��Ŀ����
��18.4mol-L-1��ŨH2SO4����100mLŨ��Ϊ1mol-L-1��H2SO4��������ɷ�Ϊ���¸�����
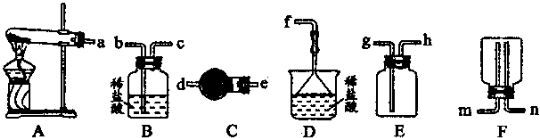
��A������Ͳȡ______mLŨH2SO4����ע��װ��Լ50mL����ˮ���ձ��У����ò��������Ͻ���
��B����Լ30mL����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴҺ������100mL����ƿ��
��C����ϡ�ͺ�H2SO4С�ĵ���100mL����ƿ��
��D�����100mL����ƿ�ڲ��Ƿ�ᷢ����©
��E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ����ο̶���______��
��F���ǽ�ƿ���������ߵ���ҡ����Һ
��G����______������ƿ����ε�������ˮ����Һ����͵�ǡ�úͻ��ο̶�������
��1�������������Ŀհ״�����������
��2����ȷ�IJ���˳���ǣ���ĸ��д��______��
��3������A������Ӧѡ��������������10mL��Ͳ����50mL��Ͳ��500mL��Ͳ����1000mL��Ͳ�еģ�����ţ�______��
��A������Ͳȡ______mLŨH2SO4����ע��װ��Լ50mL����ˮ���ձ��У����ò��������Ͻ���
��B����Լ30mL����ˮ���ֳ�����ϴ���ձ��Ͳ���������ÿ��ϴҺ������100mL����ƿ��
��C����ϡ�ͺ�H2SO4С�ĵ���100mL����ƿ��
��D�����100mL����ƿ�ڲ��Ƿ�ᷢ����©
��E��������ˮֱ�Ӽ�������ƿ����Һ��ӽ����ο̶���______��
��F���ǽ�ƿ���������ߵ���ҡ����Һ
��G����______������ƿ����ε�������ˮ����Һ����͵�ǡ�úͻ��ο̶�������
��1�������������Ŀհ״�����������
��2����ȷ�IJ���˳���ǣ���ĸ��д��______��
��3������A������Ӧѡ��������������10mL��Ͳ����50mL��Ͳ��500mL��Ͳ����1000mL��Ͳ�еģ�����ţ�______��
��1��������Һϡ��ǰ�����ʵ��������������Ũ��������������ҪŨ��������ΪV��18.4mol?L-1��V=0.1L��1mol?L-1��v=0.0054L=5.4mL��������ˮֱ�Ӽ�������ƿ����Һ��ӽ����ο̶���1��2cm�������ý�ͷ�ι�������ƿ����ε�������ˮ����Һ����͵�ǡ�úͿ̶������У�
�ʴ�Ϊ��5.4��1��2cm����ͷ�ιܣ�
��2�����������м������ƿ�Ƿ�©ˮ����ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ����D����A�� ��C�� ��B�� ��E����G�� ��F����
��3����ȡ��Ũ����������5.4mL��ѡ����Ͳ�Ĺ��Ӧ�Դ�����ȡҺ������������ѡ10mL����Ͳ���ʴ�Ϊ���٣�
�ʴ�Ϊ��5.4��1��2cm����ͷ�ιܣ�
��2�����������м������ƿ�Ƿ�©ˮ����ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ����D����A�� ��C�� ��B�� ��E����G�� ��F����
��3����ȡ��Ũ����������5.4mL��ѡ����Ͳ�Ĺ��Ӧ�Դ�����ȡҺ������������ѡ10mL����Ͳ���ʴ�Ϊ���٣�

��ϰ��ϵ�д�
�����Ŀ
��18.4mol?L-1��Ũ��������4mol?L-1��������Һʱ������������������ҺŨ��ƫ�ߵ��ǣ�������
| A����ϡ�͵�������Һת��������ƿ��δϴ���ձ��Ͳ����� | B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�� | C���ý�ͷ�ιܼ�ˮʱ�������ӹ۲���Һ��Һ��������ƿ�̶����� | D���ý�ͷ�ι�������ƿ�м�ˮʱ��Һ�����������ƿ�̶ȣ���ʱ�����õιܽ�ƿ��Һ��������ʹ��Һ��Һ����̶����� |