��Ŀ����
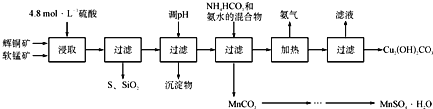
����Ŀ����������(NOCl)���л��ϳ��е���Ҫ�Լ�����ҵ�Ͽ���NO��Cl2��Ӧ�Ƶã��ش���������:
(1)NOCl�����и�ԭ�Ӿ�����8�����ȶ��ṹ����NOCl�ĵ���ʽΪ_______
(2)���������������ڴ����еĺ������������ʱ�����������������漰���·�Ӧ:
2NO2(g)+NaCl(s)![]() NaNO3(s)+NOCl(g)����H1 K1��
NaNO3(s)+NOCl(g)����H1 K1��
4NO2(g)+2NaCl(s)![]() 2NaNO3(s)+2NO(g)+Cl2(g)����H2 K2;
2NaNO3(s)+2NO(g)+Cl2(g)����H2 K2;
2NO(g)+Cl2(g)![]() 2NOCl(g)����H3 K3;
2NOCl(g)����H3 K3;
��H3=_____(�æ�H1�ͦ�H2��ʾ)��K3=______(��K1��K2��ʾ)
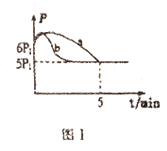
(3)25��ʱ����2L����ѹ�Ƶĺ����ܱ�������ͨ��0.08molNO��0.04molCl2������Ӧ:2NO(g)+Cl2(g)=2NOCl(g)��H
�ٲ����ѹǿ(p)��ʱ��(t)�ı仯��ͼI����a��ʾ(��Ӧ�ﵽƽ��ʱ���¶�����ʼ�¶���ͬ)����H___0(�>����<��);������������ͬ�����ı�ijһ����ʱ�������ѹǿ(p)��ʱ��(t)�ı仯��ͼI����b��ʾ����ı��������______��
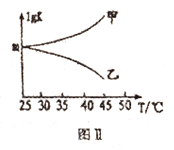
��ͼ���Ǽס���ͬѧ���������Ӧƽ�ⳣ���Ķ���ֵ(lgK)���¶ȵı仯��ϵ��������ȷ��������______ (��ס����ҡ�);mֵΪ_______
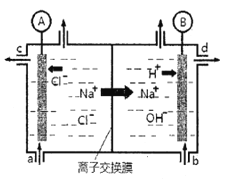
(4)NO���ü�ӵ绯ѧ����ȥ����ԭ����ͼ3��ʾ
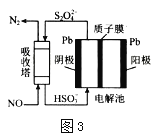
�������ĵ缫��ӦʽΪ_________
���������ڷ�����Ӧ�����ӷ���ʽΪ___________
���𰸡� ![]() 2��H1-��H2 K21/K2 < �Ӵ��� �� 2 2HSO3-+2e-+2H+=S2O42-+2H2O 2NO+2S2O42-+2H2O=N2+4HSO3-
2��H1-��H2 K21/K2 < �Ӵ��� �� 2 2HSO3-+2e-+2H+=S2O42-+2H2O 2NO+2S2O42-+2H2O=N2+4HSO3-
��������(1)NOClΪ���ۻ���������и�ԭ�Ӿ�����8�����ȶ��ṹ����NOCl�ĵ���ʽΪ![]() ����ȷ����
����ȷ����![]() ��
��
(2)���������������ڴ����еĺ������������ʱ�����������������漰���·�Ӧ:
��2NO2(g)+NaCl(s)![]() NaNO3(s)+NOCl(g)��H1 K1����4NO2(g)+2NaCl(s)
NaNO3(s)+NOCl(g)��H1 K1����4NO2(g)+2NaCl(s)![]() 2NaNO3(s)+2NO(g)+Cl2(g)����H2 K2�����ݸ�˹���ɣ��١�2-���ɵã�2NO��g��+Cl2��g��2ClNO��g�����÷�Ӧ��H3=2��H1-��H2������K1=c(NOCl)/c2(NO2)�� �� K2=c(Cl2)��c2(NO)/c4(NO2)��K3=c2(NOCl)/c(Cl2)��c2(NO),��֮��K3= K21/K2����ȷ�𰸣�2��H1-��H2��K21/K2��
2NaNO3(s)+2NO(g)+Cl2(g)����H2 K2�����ݸ�˹���ɣ��١�2-���ɵã�2NO��g��+Cl2��g��2ClNO��g�����÷�Ӧ��H3=2��H1-��H2������K1=c(NOCl)/c2(NO2)�� �� K2=c(Cl2)��c2(NO)/c4(NO2)��K3=c2(NOCl)/c(Cl2)��c2(NO),��֮��K3= K21/K2����ȷ�𰸣�2��H1-��H2��K21/K2��
(3)����ͼ1��������֪�����淴Ӧ�Ľ���ѹǿ��������С��5min�ﵽƽ��״̬����֪��ʼ��Ӧ�Ƿ��ȵģ��淴Ӧ�����¶����ߣ�ѹǿ����Ӧ��һ���̶ȣ���Ӧ��Ũ�ȼ�С���淴Ӧ������У�ѹǿ������С����ѹǿ��ʱ��仯����ʱ���ﵽƽ��״̬����Ӧ�ʱ�Ϊ����H��0���ı��ѹǿ���䣬��Ӧ���ʼӿ죬ƽ�ⲻ�ƶ�����˿���ʹ�üӴ�������ȷ�𰸣�<���Ӵ�����
����H3<0�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����С��lgK ���¶����߶���С��������ȷ������ͼ��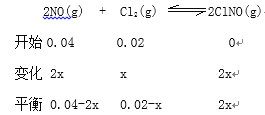
![]() X=0.01
X=0.01 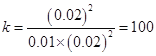 , lgK=2����ȷ�𰸣��ң�2��
, lgK=2����ȷ�𰸣��ң�2��
(4)����ͼ��֪����������HSO3-��õ�������S2O42-�����������»�����ˮ���缫��ӦʽΪ��2HSO3-+2e-+2H+=S2O42-+2H2O����ȷ�𰸣�2HSO3-+2e-+2H+=S2O42-+2H2O��
�ڣ����ճ���S2O42-��NO��Ӧ����N2��HSO3-����Ӧ���ӷ���ʽΪ2NO+2S2O42-+2H2O=N2+4HSO3-����ȷ�𰸣�2NO+2S2O42-+2H2O=N2+4HSO3-��