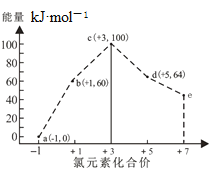
��Ŀ����
����Ŀ��ӡˢ��·���ڿƼ�������в�����������ã������Ʊ�����Ϊ�߷��ӻ������ͭ��ѹ�ϣ�ͨ��FeCl3��Һ����ʴ�����ɡ�ijʵ��С����ʵ�����÷�����ӡˢ��·��͡���ʴҺ����ȡͭ��һ�ֹ����������£�
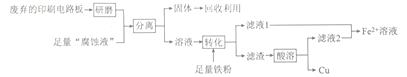
��ش��������⣺
(1) ���顰��ʴҺ���к���Fe2+�ķ���Ϊ_________________��
(2)�����롱���õIJ�������Ϊ____________���ò������õ���Ҫ�����������ձ���_____________��
(3)�õ�ⷨ�����϶�ͭʱ����������Ϊ_____________���ѧʽ���������ϵ�ƹ����е������Һ��Ũ��_______________ (�������С�����䡱����
(4) Fe2+��Һ�кܶ���Ҫ��;��
����֪�������£�Ksp[Fe(OH)2]=1.8��10-16������1.8mol��L-1��FeSO4��Һʱ��Ϊȷ����Һ�в����ֻ��ǣ�Ӧ������Һ��pH������_________________��
��Fe2+��ʹAg+��Fe3+֮���ת����һ���¶��£�0.1 mol��L-1��Fe(NO3)2��Һ�У�c(Fe3+)��c(Ag+)�Ĺ�ϵ��ͼ��ʾ��
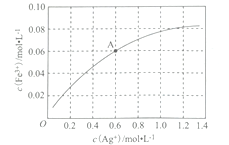
���¶��£�A����Һ��ת����Ӧ��ƽ�ⳣ��Ϊ____________(��Һ����仯���Բ��ƣ�������0.6mol��L-1 Fe(NO3)2��Һ��0.15 mol��L-1 Fe(NO3)3��Һ��0.06 mol L-1 AgNO3��Һ�������Ϻ��ټ���1.08 g Ag,�ɹ۲쵽������Ϊ_________________________��
��Fe2+ת��ΪFe3+�������Ʊ��������Ρ���FeCl3��Һ�м���NaOH��NaClO��Һ�Ʊ�Na2FeO4�Ļ�ѧ����ʽΪ_________________________��
���𰸡� ȡ��������ʴҺ���ڽྻ���Թ��У��μ�K3[Fe(CN)6]��Һ������������ɫ��������Fe2+ ���� ©���������� Fe ���� 6 2. 5 Ag�����ܽ⣬��Һ��ɫ��dz 2FeCl3+3NaClO+l0NaOH=2Na2FeO4 +9NaCl+5H2O
����������1���ں��������ӡ��������Ӻ�ͭ���ӵĻ����Һ�м����������ӵĴ��ڣ��Ϻ������Լ�Ϊ���軯����Һ����ʴҺ���к���Fe2+�ķ���Ϊ��ȡ��������ʴҺ���ڽྻ���Թ��У��μ�K3[Fe(CN)6]��Һ������������ɫ��������Fe2+����2����������ͼ�е�3��֪���������Ϊ���ˣ��������õ���Ҫ�����������ձ���©��������������3�����ʱ���Ƽ�Ϊ��������������϶�ͭ�������������������ʱ�������Һ��Ũ�������ϱ��ֲ��䡣��4����c(Fe2��)��c2(OH��)��Ksp[Fe(OH)2]��1.8��10��16ʱ������ֻ��ǣ���c(OH��)��10��8mol/L����������Ũ����10��6mol/L��pH��6������ͼ�����ݿ�֪�����¶��£�A����Һ��c(Ag��)��c(Fe3+)��0.6mol/L�����ݷ���ʽFe2+��Ag��![]() Fe3+��Ag��֪c(Fe2+)��0.04mol/L����K��0.06/(0.04��0.6)��2.5���������������ݿɵ�Ũ���أ�0.05/(0.2��0.02)��12.5��K�����Է�Ӧ������У����ʵ��������Ag�����ܽ⣬��Һ��ɫ��dz������Ԫ�ػ��ϼ۴�+3�����ߵ�+6�ۣ�ʧȥ3�����ӣ�ClԪ�ػ��ϼ۴�+1�۽��͵���1�ۣ��õ�2�����ӣ����ݵ��ӵ�ʧ�غ㡢����غ��ԭ���غ��֪�Ʊ�Na2FeO4�Ļ�ѧ����ʽΪ2FeCl3+3NaClO+l0NaOH��2Na2FeO4 +9NaCl+5H2O��
Fe3+��Ag��֪c(Fe2+)��0.04mol/L����K��0.06/(0.04��0.6)��2.5���������������ݿɵ�Ũ���أ�0.05/(0.2��0.02)��12.5��K�����Է�Ӧ������У����ʵ��������Ag�����ܽ⣬��Һ��ɫ��dz������Ԫ�ػ��ϼ۴�+3�����ߵ�+6�ۣ�ʧȥ3�����ӣ�ClԪ�ػ��ϼ۴�+1�۽��͵���1�ۣ��õ�2�����ӣ����ݵ��ӵ�ʧ�غ㡢����غ��ԭ���غ��֪�Ʊ�Na2FeO4�Ļ�ѧ����ʽΪ2FeCl3+3NaClO+l0NaOH��2Na2FeO4 +9NaCl+5H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�