��Ŀ����
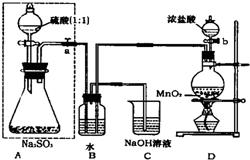 ���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�����ⶨ���������Լ��ı������ij̶ȣ��������ͼʵ�飺��ش���������⣺
���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�����ⶨ���������Լ��ı������ij̶ȣ��������ͼʵ�飺��ش���������⣺��1���������߿��ڵķ�Һ©�����ɳ���©������Ӧ������������߿���װ�õ������ԣ�
�رյ��ɼУ���ֹˮ�У�a���ɳ���©������ƿ�м�ˮ��©����Һ�������ƿ��Һ�棬��һ��ʱ��۲�Һ���Ƿ�仯�������䣬˵�������Ժã�����˵��װ��©��
�رյ��ɼУ���ֹˮ�У�a���ɳ���©������ƿ�м�ˮ��©����Һ�������ƿ��Һ�棬��һ��ʱ��۲�Һ���Ƿ�仯�������䣬˵�������Ժã�����˵��װ��©��
����2��Dװ���з�Ӧ�Ļ�ѧ����ʽΪ
MnO2+4HCl��Ũ��
MnCl2+Cl2��2H2O
| ||
MnO2+4HCl��Ũ��
MnCl2+Cl2��2H2O
Bװ���з�Ӧ�����ӷ���Ϊ
| ||
Cl2+SO2+2H2O�T4H++2Cl-+SO42-
Cl2+SO2+2H2O�T4H++2Cl-+SO42-
����3������ag Na2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м���������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����ð�ɫ����bg��ԭ��Ʒ��Na2SO3����������Ϊ
| 126b |
| 233a |
| 126b |
| 233a |
��4��Ϊ��֤ʵ��ⶨ��ȷ�ԣ���Cװ���з�Ӧ�����ӷ���ʽΪ��
Cl2+2OH-�TCl-+ClO-+2H2O
Cl2+2OH-�TCl-+ClO-+2H2O
����5�����������Լ���������ˮ����ϡ���ᡢ��ϡ���ᡢ��BaCl2��Һ����Ba��NO3��2��Һ�������ѡ������Լ���
�٢ڢ�
�٢ڢ�
���һ�ֲ�ͬ��ʵ�鷽���ⶨ��������ˮ�������Ʊ������ij̶�һ��������Na2SO3��Ʒ���Թ��У���������������ˮ�ܽ⣬���������ϡ���ᣬ���ٲ������ݣ��ټ���������BaCl2��Һ��ַ�Ӧ�����ˣ�ϴ�ӣ�������أ��������������Na2SO3�����������������ˮ�������Ʊ������İٷֺ�����
һ��������Na2SO3��Ʒ���Թ��У���������������ˮ�ܽ⣬���������ϡ���ᣬ���ٲ������ݣ��ټ���������BaCl2��Һ��ַ�Ӧ�����ˣ�ϴ�ӣ�������أ��������������Na2SO3�����������������ˮ�������Ʊ������İٷֺ�����
����������1������©��û�����ӣ������ˮ��©����Һ�������ƿ��Һ�棬�γ��ܱ�ϵͳ���γ�ѹǿ��۲�Һ��仯��
��2��DΪʵ�����Ʊ������ķ���װ�ã��������������ԣ�����������л�ԭ�ԣ���B�з���������ԭ��Ӧ������������
��3��������Ԫ���غ���㣬Na2SO3��SO2��BaSO4��
��4��C��ΪNaOH��Һ������������Һ�з�Ӧ����NaCl��NaClO��
��5���������Ʊ�����Ϊ�����ƣ������Ȼ����������ᱵ�������Դ˼���ٷֺ�����
��2��DΪʵ�����Ʊ������ķ���װ�ã��������������ԣ�����������л�ԭ�ԣ���B�з���������ԭ��Ӧ������������
��3��������Ԫ���غ���㣬Na2SO3��SO2��BaSO4��
��4��C��ΪNaOH��Һ������������Һ�з�Ӧ����NaCl��NaClO��
��5���������Ʊ�����Ϊ�����ƣ������Ȼ����������ᱵ�������Դ˼���ٷֺ�����
����⣺��1������ʵ��װ�õ������Գ��õ������Ǽ��Ȼ��γ�Һ�����γ�ѹǿ�����۲��Ƿ������ݻ�Һ���Ƿ����仯������©��û�����ӣ������ˮ��©����Һ�������ƿ��Һ�棬�γ��ܱ�ϵͳ���γ�ѹǿ��۲�Һ��仯��
�ʴ�Ϊ���رյ��ɼУ���ֹˮ�У�a���ɳ���©������ƿ�м�ˮ��©����Һ�������ƿ��Һ�棬��һ��ʱ��۲�Һ���Ƿ�仯�������䣬˵�������Ժã�����˵��װ��©����
��2��DΪʵ�����Ʊ������ķ���װ�ã���ӦΪ��MnO2+4HCl��Ũ��
MnCl2+Cl2��2H2O���������������ԣ�����������л�ԭ�ԣ���B�з���������ԭ��Ӧ������������ᣬ��ӦΪ��Cl2+SO2+2H2O�T4H++2Cl-+SO42-���ʴ�Ϊ��MnO2+4HCl��Ũ��
MnCl2+Cl2��2H2O��Cl2+SO2+2H2O�T4H++2Cl-+SO42-��
��3��������Ԫ���غ���㣬
Na2SO3��SO2��BaSO4
126g 233g
m bg
m=
g������Na2SO3����������Ϊ
��100%���ʴ�Ϊ��
��100%��
��4��������NaOH��Һ�з�Ӧ����NaCl��NaClO����ӦΪ��Cl2+2OH-�TCl-+ClO-+2H2O���ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+2H2O��
��5�����������л��������ƣ����ʵ�鷽��ʱ�ɴ������Ƕ���ƣ�һ�Dzⶨ�������Ƶĺ��������Dzⶨ�����Ƶĺ������������Ʊ�����Ϊ�����ƣ������Ȼ����������ᱵ�������������ᱵ��������������Ƶ��������ɼ�����������Ƶ����������дӵ�һ���Ƕ���ƣ�����ɴӵڶ����Ƕ���ƣ����������ȥ�������ƣ������Ȼ����������ᱵ�������������ᱵ��������������Ƶ��������ɼ�����������Ƶ�������
�ʴ�Ϊ���٢ڢܣ�һ��������Na2SO3��Ʒ���Թ��У���������������ˮ�ܽ⣬���������ϡ���ᣬ���ٲ������ݣ��ټ���������BaCl2��Һ��ַ�Ӧ�����ˣ�ϴ�ӣ�������أ��������������Na2SO3�����������������ˮ�������Ʊ������İٷֺ�����
�ʴ�Ϊ���رյ��ɼУ���ֹˮ�У�a���ɳ���©������ƿ�м�ˮ��©����Һ�������ƿ��Һ�棬��һ��ʱ��۲�Һ���Ƿ�仯�������䣬˵�������Ժã�����˵��װ��©����
��2��DΪʵ�����Ʊ������ķ���װ�ã���ӦΪ��MnO2+4HCl��Ũ��
| ||
| ||
��3��������Ԫ���غ���㣬
Na2SO3��SO2��BaSO4
126g 233g
m bg
m=
| 126b |
| 233 |
| 126b |
| 233a |
| 126b |
| 233a |
��4��������NaOH��Һ�з�Ӧ����NaCl��NaClO����ӦΪ��Cl2+2OH-�TCl-+ClO-+2H2O���ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+2H2O��
��5�����������л��������ƣ����ʵ�鷽��ʱ�ɴ������Ƕ���ƣ�һ�Dzⶨ�������Ƶĺ��������Dzⶨ�����Ƶĺ������������Ʊ�����Ϊ�����ƣ������Ȼ����������ᱵ�������������ᱵ��������������Ƶ��������ɼ�����������Ƶ����������дӵ�һ���Ƕ���ƣ�����ɴӵڶ����Ƕ���ƣ����������ȥ�������ƣ������Ȼ����������ᱵ�������������ᱵ��������������Ƶ��������ɼ�����������Ƶ�������
�ʴ�Ϊ���٢ڢܣ�һ��������Na2SO3��Ʒ���Թ��У���������������ˮ�ܽ⣬���������ϡ���ᣬ���ٲ������ݣ��ټ���������BaCl2��Һ��ַ�Ӧ�����ˣ�ϴ�ӣ�������أ��������������Na2SO3�����������������ˮ�������Ʊ������İٷֺ�����
��������ʵ��Ƚϳ��棬Ӧ��˵�ǿ������֪ʶ�����ճ̶ȣ���1������װ�������Լ��ķ�������2������β����������3������ʽ��д����4������ʵ�����������

��ϰ��ϵ�д�
�����Ŀ
 ���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�������ԡ������ⶨ���������Լ��ı������ij̶ȣ�������·�����
���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�������ԡ������ⶨ���������Լ��ı������ij̶ȣ�������·�����