��Ŀ����
ʵ���ҿ���̼�����Ʊ������������ƾ��壬�й�װ������ͼ��ʾ��

��17 g Na2CO3����80 mLˮ�У���װ��C��D������ƿ�У��ٽ�25 gͭм����Բ����ƿ�У���60 mLŨH2SO4��10 mLˮ�Ļ��Һװ�ڷ�Һ©���У���μ��������С�ļ���ʹSO2����(ע�����������С�ͷ�Ӧ���ʵĿ���)����SO2ͨ��Na2CO3��Һ�������ͣ��ںϲ�C��D��ƿ������Һ�У���������17 g Na2CO3������Ũ������ȴ����������ƾ��塣�Իش�
(1)�������60 mLŨH2SO4��10 mLˮ�Ļ��Һ��
(2)װ��B��ŨH2SO4�����ã�______________________________________________________��
(3)Na2SO3��ҺҪ��װ��������ƿ�е�������__________________��д��C�з�����Ӧ�����ӷ���ʽ__________________��
(4)����SO2�����ٶȵķ�����_____________________________________________��
(5)ͨSO2������Na2CO3��Ŀ����____________________________________��

��17 g Na2CO3����80 mLˮ�У���װ��C��D������ƿ�У��ٽ�25 gͭм����Բ����ƿ�У���60 mLŨH2SO4��10 mLˮ�Ļ��Һװ�ڷ�Һ©���У���μ��������С�ļ���ʹSO2����(ע�����������С�ͷ�Ӧ���ʵĿ���)����SO2ͨ��Na2CO3��Һ�������ͣ��ںϲ�C��D��ƿ������Һ�У���������17 g Na2CO3������Ũ������ȴ����������ƾ��塣�Իش�
(1)�������60 mLŨH2SO4��10 mLˮ�Ļ��Һ��
(2)װ��B��ŨH2SO4�����ã�______________________________________________________��
(3)Na2SO3��ҺҪ��װ��������ƿ�е�������__________________��д��C�з�����Ӧ�����ӷ���ʽ__________________��
(4)����SO2�����ٶȵķ�����_____________________________________________��
(5)ͨSO2������Na2CO3��Ŀ����____________________________________��
(1)ȡ10 mL����ˮ�����ձ��У����ձ��ڽ�60 mLŨH2SO4����ע��ˮ�У�ͬʱ���Ͻ��衣
(2)����ҿɸ��ݷų�����Ŀ����̶��ж�SO2�������ٶ�
(3)�������SO2 2SO2+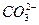 +H2O====CO2��+
+H2O====CO2��+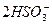
(4)�۲�B�г�������Ŀ�����ͨ�����ȿ��Ʒ�Ӧ�ٶȣ���B������ų����죬����ȥ�ƾ��ƣ������ͺ�����С�����
(5)ʹNaHSO3ת��ΪNa2SO3
(2)����ҿɸ��ݷų�����Ŀ����̶��ж�SO2�������ٶ�
(3)�������SO2 2SO2+
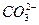 +H2O====CO2��+
+H2O====CO2��+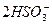
(4)�۲�B�г�������Ŀ�����ͨ�����ȿ��Ʒ�Ӧ�ٶȣ���B������ų����죬����ȥ�ƾ��ƣ������ͺ�����С�����
(5)ʹNaHSO3ת��ΪNa2SO3
ŨH2SO4Ϊ�������ᣬ��Cu��Ӧ����SO2��SO2��ˮ��ҺΪH2SO3��H2SO3�����Ա�H2CO3ǿ��SO2��Na2CO3��Һ���ÿɲ���Na2SO3��CO2,�䷴Ӧ����ʽ�ֱ�ΪCu+2H2SO4(Ũ)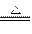 CuSO4+SO2��+2H2O,SO2+Na2CO3====Na2SO3+CO2����
CuSO4+SO2��+2H2O,SO2+Na2CO3====Na2SO3+CO2����
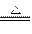 CuSO4+SO2��+2H2O,SO2+Na2CO3====Na2SO3+CO2����
CuSO4+SO2��+2H2O,SO2+Na2CO3====Na2SO3+CO2����
��ϰ��ϵ�д�
�����Ŀ
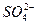 ====BaSO4��
====BaSO4��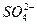 ��
��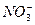 ��
��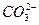 ��
��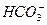 ���ֽ�������ʵ�飺
���ֽ�������ʵ�飺