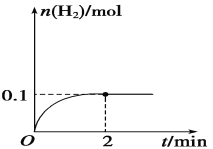
��Ŀ����
����Ŀ���������ʿ���ͨ������Ǩ�ƴ��ݵ�ɣ����ù�������RbAg4I5�����Ƴɵ绯ѧ����������������Ǩ�Ƶ�����ȫ��Ag+����ͼ��һ�ֲⶨO2���������崫����ʾ��ͼ��O2���������ķ���ϩ��Ĥ�����ݵ�ص綯�Ʊ仯���Բ��O2�ĺ����������崫�������������У������й�˵����ȷ����

A.���缫�����ģ�RbAg4I5��������
B.��λ�ƶ���Խ��O2����Խ��
C.������ӦΪAg+I--e-= AgI
D.����A1I3ͬ���ΪAl��AgI
���𰸡�B
��������
O2ͨ�������Ӧ��4AlI3+3O2=2Al2O3+6I2��I2�ڴ���������з�����ԭ��Ӧ����˶��ʯī�缫Ϊ�������缫��ӦʽΪ��I2+2Ag++2e-=2AgI�����缫����������Ӧ��������������������RbAg4I5��Ǩ�Ƶ�������Ag+���缫��ӦʽΪ��Ag-e-=Ag+���ݴ˽��
A��������������֪�����������������У����缫�����ģ����������ܷ�ӦΪ��I2+2Ag=2AgI�����������RbAg4I5��Ǩ�Ƶ�������Ag+�����RbAg4I5�������ᷢ���仯����A����
B��O2����Խ�ߣ���λʱ����ת�Ƶ�����Խ�࣬��λ�ƶ���Խ��B��ȷ��
C��������������֪��������ӦΪ��Ag-e-=Ag+����C����
D��������������֪������AlI3����Al2O3��I2����D����
�ʴ�Ϊ��B��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�