��Ŀ����
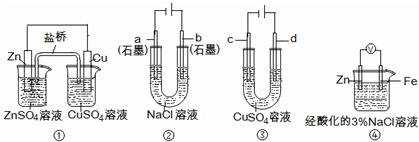
��ͼ��ʾ2��ʵ��װ�ã��ֱ�ش��������⣮

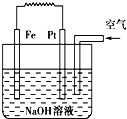
��1��װ��1�е�Cu��______�������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ______��
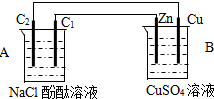
��2��װ��2�м��ձ�ʢ��100mL0.2mol/L��NaCl��Һ�����ձ�ʢ��100mL0.5mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽�ұ�ʯī�缫�������ȱ�죬���ʯī�缫��������������
�ٵ�Դ��M��Ϊ______�������ձ��ұ�ʯī�缫�������ȱ���ԭ����______��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ______��
����װ�ü�����������������112mL���壨��״��������װ����������Һ��pHΪ______�����Է�Ӧǰ����Һ������仯����

��1��װ��1�е�Cu��______�������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ______��
��2��װ��2�м��ձ�ʢ��100mL0.2mol/L��NaCl��Һ�����ձ�ʢ��100mL0.5mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽�ұ�ʯī�缫�������ȱ�죬���ʯī�缫��������������
�ٵ�Դ��M��Ϊ______�������ձ��ұ�ʯī�缫�������ȱ���ԭ����______��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ______��
����װ�ü�����������������112mL���壨��״��������װ����������Һ��pHΪ______�����Է�Ӧǰ����Һ������仯����
��1��װ��1Ϊԭ��أ�ͭΪ��������������ʯī��������ط�ӦΪ2Fe3++Cu�T2Fe2++Cu2+���ʴ�Ϊ������2Fe3++Cu�T2Fe2++Cu2+��
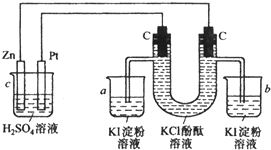
��2������ձ��е��뼸�η�̪���۲쵽�ұ�ʯī�缫�������ȱ�죬˵�������ұߵ缫����OH-��ӦΪ���ص���������MΪ��Դ��������NΪ��Դ�ĸ�������Ϊ���ʳ��ˮװ�ã���Ϊ�������ͭװ�ã���
�������Ϸ�����֪MΪ�����������Ϸֱ���H2O?H++OH-��2H++2e-�TH2����c��OH-����c��H+������Һ�ʼ��ԣ�
�ʴ�Ϊ������H2O?H++OH-��H++2e-�TH2����c��OH-����c��H+������Һ�ʼ��ԣ�
����Ϊ�������ͭ��Һ������������������������ͭ�����ķ���ʽΪ2Cu2++2H2O
2Cu+O2��+4H+���ʴ�Ϊ��2Cu2++2H2O
2Cu+O2��+4H+��
����װ�ü�����������������112mL���壨��״��������n��H2��=
=0.005mol����2H++2e-�TH2����֪ת��0.01mol���ӣ���2Cu2++2H2O
2Cu+O2��+4H+��֪������0.01molH+����c��H+��=
=0.1mol/L��pH=1��
�ʴ�Ϊ��1��
��2������ձ��е��뼸�η�̪���۲쵽�ұ�ʯī�缫�������ȱ�죬˵�������ұߵ缫����OH-��ӦΪ���ص���������MΪ��Դ��������NΪ��Դ�ĸ�������Ϊ���ʳ��ˮװ�ã���Ϊ�������ͭװ�ã���
�������Ϸ�����֪MΪ�����������Ϸֱ���H2O?H++OH-��2H++2e-�TH2����c��OH-����c��H+������Һ�ʼ��ԣ�
�ʴ�Ϊ������H2O?H++OH-��H++2e-�TH2����c��OH-����c��H+������Һ�ʼ��ԣ�
����Ϊ�������ͭ��Һ������������������������ͭ�����ķ���ʽΪ2Cu2++2H2O
| ||
| ||
����װ�ü�����������������112mL���壨��״��������n��H2��=
| 0.112L |
| 22.4L/mol |
| ||
| 0.01mol |
| 0.1L |
�ʴ�Ϊ��1��

��ϰ��ϵ�д�
�����Ŀ