��Ŀ����
���ж����ڵ�����Ԫ��X��Y��Z��ԭ���������α�С��ԭ�Ӻ�����Ӳ���֮��Ϊ5��XԪ��ԭ��������ϵĵ�������YԪ��ԭ���������ZԪ��ԭ������������֮�ͣ�YԪ��������ϵ�����������Ӳ�����2����X��Z�����γ�XZ3�ͻ������ش����⣺
(1) X������_________��Y������_________��Z������_________��
(2) XZ3�Ļ�ѧʽΪ________������ʽΪ______�����Ӽ����________����
(3)X����������Ӧˮ�����Ũ��Һ����ȵ�Y���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(1) X������_________��Y������_________��Z������_________��
(2) XZ3�Ļ�ѧʽΪ________������ʽΪ______�����Ӽ����________����
(3)X����������Ӧˮ�����Ũ��Һ����ȵ�Y���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________��
��7�֣�(1)����̼����(2)NH3��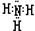 �� ��(3)C+4HNO3(Ũ)
�� ��(3)C+4HNO3(Ũ)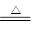 CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O
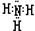 �� ��(3)C+4HNO3(Ũ)
�� ��(3)C+4HNO3(Ũ)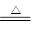 CO2��+4NO2��+2H2O
CO2��+4NO2��+2H2O���������YԪ��������ϵ�����������Ӳ�����2������X��̼Ԫ�ػ�SԪ�ء���������Ԫ�ص�ԭ�Ӻ�����Ӳ���֮��Ϊ5������Y�������ǵ������ڵ�Ԫ�أ���Y��̼Ԫ�أ���X��Z��Ӧ���ǵ�һ���ں͵ڶ����ڵ�Ԫ�أ�����ԭ��������֪��Zһ������Ԫ�ء�XԪ��ԭ��������ϵĵ�������YԪ��ԭ���������ZԪ��ԭ������������֮�ͣ�X��Z�����γ�XZ3�ͻ��������X�ǵ�Ԫ�ء�
�����������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ������������ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ