��Ŀ����
����Ŀ����ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ��
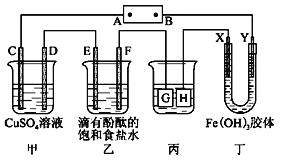
��ش𣺣�1��B���ǵ�Դ��___�����缫C�ĵ缫��ӦʽΪ��___��
��2����C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ___��
��3�����ñ�װ�ø�ͭ����������H�缫������___���ͭ���������������Һ��___��Һ����������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ___��
��4��һ��ʱ�����X����������ɫ��dz��Y����������ɫ��������__�ڵ糡��������Y���ƶ���
���𰸡����� 4OH-��4e-=O2��+2H2O 1��2��2��2 Cu AgNO3 5.4g ������������
��������
��װ��ͼ��֪��ֱ����Դ��ͨ��F�������ʺ�ɫ˵��F��Ϊ������E��Ϊ������D��Ϊ������C��Ϊ������Y��Ϊ������X��Ϊ������H��Ϊ������G��Ϊ������
��1��Y�����������Դ�ĸ��������ӣ����Դ�����������ӵ��ǵ��ص�����������������Ӧ��
��2��C��D��E��F�缫ת�Ƶĵ�����Ŀ��ȣ�����ת�Ƶĵ��ӵ����ʵ����������ɵ��ʵ����ʵ�����
��3���ñ�װ�ø�ͭ������ʱ�����ƽ���λ���������Ʋ����λ��������
��4��һ��ʱ�����X����������ɫ��dz��Y����������ɫ���˵�������˽���ĵ�Ӿ����
��1��Y�����������Դ�ĸ��������ӣ����BΪ��Դ��������CΪ���ص�����������������Ӧ���缫��ӦΪ��4OH-��4e-=O2��+2H2O��
��2��C��D��E��F�缫�����ĵ缫��Ӧ�ֱ�Ϊ��4OH-��4e-=O2��+2H2O��Cu2++2e-=Cu��2Cl--2e-=Cl2����2H++2e-=H2���������缫ת�Ƶĵ��Ӿ�Ϊ1molʱ�����ɵĵ��ʷֱ�Ϊ0.25mol��0.5mol��0.5mol��0.5mol�����ʵ���֮��Ϊ1:2:2:2��
��3���ñ�װ�ø�ͭ������ʱ�����ƽ���λ���������Ʋ����λ���������������H����ͭ��������G���ý����������Һ����Ag+���ɣ���AgNO3������������Һ��pH��13ʱ������2H++2e-=H2����֪�ŵ�������ӵ����ʵ���Ϊ0.1mol/L��0.5L=0.05mol��������ת��0.05molʱ��������0.05mol��108g/mol=5.4g��
��4��һ��ʱ�����X����������ɫ��dz��Y����������ɫ���˵�������˽���ĵ�Ӿ�����������������ڵ糡���������������ƶ���

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ�������±���25��ʱijЩ����ĵ���ƽ�ⳣ����
��ѧʽ | CH3COOH | HClO | H2CO3 | H2C2O4 |
Ka | Ka��1.8��10��5 | Ka��3.0��10��8 | Ka1��4.1��10��7 Ka2��5.6��10��11 | Ka1��5.9��10��2 Ka2��6.4��10��5 |
��1��H2C2O4�뺬�����ʵ�����KOH����Һ��Ӧ��������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ___��
��2��pH��ͬ��NaClO��CH3COOK��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ��(����>������<����������)��CH3COOK___NaClO������Һ�У�[c(Na��)��c(ClO��)]___[c(K��)��c(CH3COO��)]��
��3����0.1mol��L��1CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO��)��5��9����ʱ��ҺpH��___��
��4��̼������Һ�еμ�������ˮ�����ӷ���ʽΪ___��
����H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
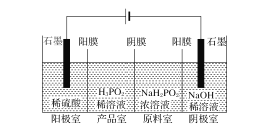
��1��д�������ĵ缫��Ӧʽ___��
��2��������Ʒ�ҿɵõ�H3PO2��ԭ��___��
����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g��![]() CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��___��
��2���÷�ӦΪ___��Ӧ��ѡ�����ȡ����ȣ���
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������___����ѡ�۷֣���
a��������H2����������H2O�� b����Ӧֹͣ�������淴Ӧ���ʶ�������
c��������ѹǿ���ٷ����仯 d�����������c��CO2������
��4��ij�¶��£���2L���ܱ������У�����1 molCO2��1molH2��ַ�Ӧ��ƽ��ʱ��COƽ��Ũ��Ϊ0.25mol/L�����жϴ�ʱ���¶�Ϊ__�档
��5�����ڣ�4���������¶��£���1L���ܱ������У�����2molCO2��3molH2��ַ�Ӧ��ƽ��ʱ��CO2��ƽ��ת����Ϊ___��