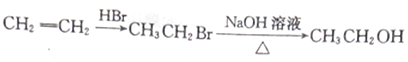题目内容
【题目】下表是元素周期表的一部分,已知⑤为短周期元素,其单质为淡黄色固体,据表回答有关问题:
① | ② | ||||||
③ | ④ | ⑤ | ⑥ | ⑦ | |||
⑧ | ⑨ |
(1)画出元素⑧的原子结构示意图 __________________
(2)在这些元素中,最活泼的非金属元素是 ______, 最不活泼的元素是_____(写元素符号 )。
(3)在这些元素的最高价氧化物对应水化物中, 碱性最强的是 __________(写化学式),呈两性的氢氧化物是____________(写化学式),写出两者之间反应的离子方程式: ______________________________
(4)在⑥与⑨中,化学性质较活泼的是________(写元素符号 ),写出可以验证该结论的一个化学反应方式 _________________________________________。
【答案】 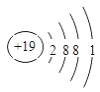 F Ar KOH Al(OH)3 Al(OH)3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
F Ar KOH Al(OH)3 Al(OH)3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
【解析】已知⑤为短周期元素,其单质为淡黄色固体,则⑤是S,所以①是Li,②是F,③是Na,④是Al,⑥是Cl,⑦是Ar,⑧是K,⑨是Br。
(1)K元素的原子结构示意图为 。(2)在这些元素中,最活泼的非金属元素是F, 最不活泼的元素是稀有气体Ar。 (3)在这些元素的最高价氧化物对应水化物中, 碱性最强的是KOH,呈两性的氢氧化物是Al(OH)3,两者之间反应的离子方程式为Al(OH)3 +OH- =AlO2- + 2H2O。(4)同主族随原子序数的增大非金属性减弱,则在⑥与⑨中,化学性质较活泼的是Cl,氯气能把溴置换出来可以验证该结论,反应的化学反应方式为 Cl2+2NaBr=Br2+2HCl。
。(2)在这些元素中,最活泼的非金属元素是F, 最不活泼的元素是稀有气体Ar。 (3)在这些元素的最高价氧化物对应水化物中, 碱性最强的是KOH,呈两性的氢氧化物是Al(OH)3,两者之间反应的离子方程式为Al(OH)3 +OH- =AlO2- + 2H2O。(4)同主族随原子序数的增大非金属性减弱,则在⑥与⑨中,化学性质较活泼的是Cl,氯气能把溴置换出来可以验证该结论,反应的化学反应方式为 Cl2+2NaBr=Br2+2HCl。

【题目】液化石油气作为燃料,已普遍进入城市家庭,它是含有下列物质的混合物,在常压下,这些物质的沸点如下表所示:
物质名称 | 乙烷 | 丙烷 | 丁烷 | 戊烷 | 己烷 |
沸点/℃ | -88.6 | -42.1 | -0.5 | 36.1 | 69.2 |
在常温下使用至无气体放出时,钢瓶中常剩余一些液态物质,这些物质最有可能是( )
A. 乙烷、丙烷和丁烷 B. 乙烷和丙烷
C. 只有乙烷 D. 戊烷和己烷