��Ŀ����
����Ŀ��ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25 g��L��1�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��

��1��A������Ϊ________��
��2��E�Ľṹ��ʽ_________________��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ���(��ͼ��ʾ)���ظ�����2��3�Ρ��÷�Ӧ�Ļ�ѧ����ʽΪ________________________��

��4����AΪԭ�ϣ���һ�������£������Ʊ��߷��ӻ�����PE�������ʿ����ڱ���Ĥ������ʳƷ������ƿ����Ͱ��ˮ���ȣ���д����Ӧ����ʽ_______���÷�Ӧ����_______����Ӧ���ͣ���
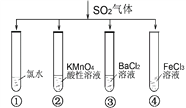
��5��B��D��Ũ�����������ʵ�ַ�Ӧ�ܣ�ʵ��װ������ͼ��ʾ����ͼ��װ���������Թ�1���ȼ���amLCH3CH2OH���ܶ�Ϊ��g/cm-3�������ı�����(CH3CO18OH)��������������2mLŨH2SO4�����Թ̶ܹ�������̨�ϣ����Թ�2�м��������ı���Na2CO3��Һ���þƾ��ƶ��Թ�1���ȣ����۲쵽�Թ�2������������ʱ��Ϊ��Ӧ������ɡ�

a���Թ�1�з�Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
b�����۲쵽�Թ�2����_____________________________����ʱ��Ϊ��Ӧ������ɡ�
c�������Թ�2�еĻ������Եõ���Ʒ������δ��Ӧ��������Ҵ���ʵ������������£�

�����ڵ�������__________________��
d�����õ���������������bg����ʵ�������������IJ���______����a��b������ʾ����
���𰸡���ϩ CH2BrCH2Br 2CH3CH2OH��O2![]() 2CH3CHO��2H2O nCH2=CH2
2CH3CHO��2H2O nCH2=CH2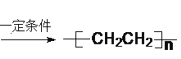 �Ӿ۷�Ӧ���ӳɷ�Ӧ��ۺϷ�Ӧ�� CH3CH2OH+CH3CO18OH
�Ӿ۷�Ӧ���ӳɷ�Ӧ��ۺϷ�Ӧ�� CH3CH2OH+CH3CO18OH ![]() CH3COOCH2CH3+H218O ��״Һ�岻������ʱ��������״Һ������ʱҲ�ɣ� ����
CH3COOCH2CH3+H218O ��״Һ�岻������ʱ��������״Һ������ʱҲ�ɣ� ���� ![]() ��
��![]()
��������
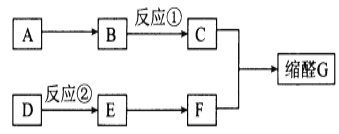
�����л���ļ��ת����ϵ�����ʵ����ʷ�������Ӧ�����ɣ���д��ط�Ӧ����ʽ�������Ʊ����������ĵ�ʵ��ԭ������ʵ���в�����ע�����
A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L����Ħ������=1.25g/L��22.4L/mo1=28g/mo1��������Ǻ���һ������ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��������C��C��������D��B��D���������г������л��D����̼�����Ʒ�Ӧ�����D�ķ���ʽ��֪��BΪCH3CHOH��CΪCH3CHO��DΪCH3COOH���ʷ�Ӧ������ϩ��ˮ�����ӳɷ�Ӧ����CH3CH2OH��CH3CH2OH��CH3COOH��Ũ���������·���������Ӧ����������������FΪCH3COOCH2CH3����ϩ���巢���ӳɷ�Ӧ���ɵ�EΪ1��2-�������顣
��1��������������֪��AΪ��ϩ���ʴ�Ϊ����ϩ��
��2��EΪ1��2-�������飬�ṹ��ʽΪCH2BrCH2Br���ʴ�Ϊ��CH2BrCH2Br��
��3����Ӧ��Ϊ�Ҵ��Ĵ�����������ȩ����Ӧ����ʽΪ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O ���ʴ�Ϊ��2CH3CH2OH��O2
2CH3CHO��2H2O ���ʴ�Ϊ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O ��
2CH3CHO��2H2O ��
��4��AΪ��ϩ����һ�������£������Ʊ��߷��ӻ��������ϩ����Ӧ����ʽΪ��nCH2=CH2![]() [CH2-CH2]n���÷�ӦΪ�Ӿ۷�Ӧ���ʴ�Ϊ��nCH2=CH2
[CH2-CH2]n���÷�ӦΪ�Ӿ۷�Ӧ���ʴ�Ϊ��nCH2=CH2![]() [CH2-CH2]n���Ӿ۷�Ӧ��
[CH2-CH2]n���Ӿ۷�Ӧ��
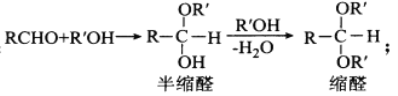
��5��a����������Ӧ�У�����ȥ�ǻ�������ȥ�⣬����18O������ˮ�У���Ӧ����ʽΪ��CH3CH2OH+CH3CO18OH ![]() CH3COOCH2CH3+H218O��
CH3COOCH2CH3+H218O��
b�����ɵ�������������״��������̼������Һ�����Ե��۲쵽�Թ�2������״Һ�岻������ʱ��������״Һ������ʱҲ�ɣ�����Ϊ��Ӧ������ɣ�
c�������ᡢ�Ҵ������������м��뱥��̼������Һ��������̼���Ʒ�Ӧ��������ˮ�������ƣ��������������ڱ���̼������Һ��������Ϊ��Һ��AΪ���Ҵ��������Ƶ���Һ�������Ҵ��ӷ������ʷ�����������Ϊ�����Խ��Ҵ����������
d����Ϊ��������������Ҵ��������ɵ���������
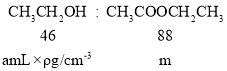
���ݹ�ϵʽ�����������Ƶ���������������Ϊ![]() g����ʵ��IJ���Ϊ��
g����ʵ��IJ���Ϊ�� ��
��
�ʴ�Ϊ��CH3CH2OH+CH3CO18OH ![]() CH3COOCH2CH3+H218O����״Һ�岻������ʱ��������״Һ������ʱҲ�ɣ�������
CH3COOCH2CH3+H218O����״Һ�岻������ʱ��������״Һ������ʱҲ�ɣ�������![]() ��
��![]() ��
��