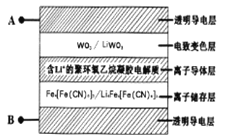
��Ŀ����
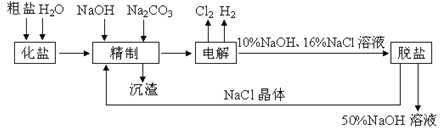
����Ŀ���ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ļ�ѧ��Ӧ����ʽΪ��2NaCl + H2O![]() 2NaOH + H2��+ Cl2������������ʾ��ͼ���£�
2NaOH + H2��+ Cl2������������ʾ��ͼ���£�
�±���NaCl��MaOH��ˮ�е��ܽ��
�¶� ���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
NaCl | 35.7g | 36g | 36.6g | 37.3g | 38.4g | 39.8g |
NaOH | 42g | 109g | 129g | 174g | 314g | 347g |
����ʾ��ͼ�ͱ������������գ�
��1����ҵʳ���к�CaCl2��MgCl2�����ʡ���ȥCa2+��Mg2+���̷�����Ӧ�Ļ�ѧ��Ӧ����ʽΪ_____��
��2�����ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��______����ȴ��______����д�������ƣ���ȥNaCl��
��3�����������SO42-�����ϸߣ��������ӱ��Լ���ȥSO42-���ñ��Լ�������________������ĸ��ţ���
��Ba(NO3)2 ��BaCl2
��4��Ϊ����Ч�س�ȥCa2+��Mg2+��SO42-�������Լ��ĺ���˳��Ϊ_______����ѡ��ѡ�����Ʒ�����
A.�ȼ�NaOH�����Na2CO3���ټӱ��Լ� B.�ȼ�Na2CO3����ӱ��Լ����ټ�NaOH
C.�ȼӱ��Լ������NaOH���ټ�Na2CO3 D.�ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
��5�����Ƶõ� NaOH�������� 240 mL0.2mol/L NaOH ��Һ��
������ʱ�����ʹ�õIJ���������______________________��
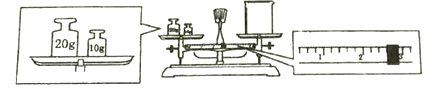
��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��
�ձ���ʵ������Ϊ________________g��
�������ƹ����У���������������ȷ�ģ�������������Ƶ�NaOH��Һ��Ũ��ƫ����_____��
A.û��ϴ���ձ��Ͳ����� B.����ƿ�����������������ˮ
C.����ʱ���ӿ̶��� D.����ʱ���ӿ̶���
E.���ݺ�����ƿ������ҡ�������ú���Һ����ڿ̶������ټ�ˮ���̶�����
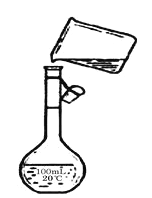
����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����_____________��
���𰸡� CaCl2+ Na2CO3=CaCO3��+2NaCl ��MgCl2+2NaOH=Mg(OH)2��+2NaCl ���� ���� �� C D ��ͷ�ιܡ�250mL����ƿ����Ͳ���ձ��������� 27. 4 C δ�ò�����������δѡ��250mL����ƿ
�������������������1����ҵʳ���к�CaCl2��MgCl2�����ʡ���ȥCa2+��Mg2+���̷�����Ӧ�Ļ�ѧ��Ӧ����ʽΪCaCl2+ Na2CO3=CaCO3��+2NaCl ��MgCl2+2NaOH=Mg(OH)2��+2NaCl��
��2�������㣬10%NaOH��ҺŨ����50%ʱ����δ�ﵽ�����µı�����Һ����Һ������Ϊԭ����![]() ������NaCl���������������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ����������ҺŨ������ȴ�����˳�ȥNaCl��
������NaCl���������������ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ����������ҺŨ������ȴ�����˳�ȥNaCl��
��3�����������SO42-�����ϸߣ��������ӱ��Լ���ȥSO42-���ñ��Լ�������BaCl2����ڣ���ѡ��Ba(NO3)2�����������������������
��4��Ϊ����Ч�س�ȥCa2+��Mg2+��SO42-��ͨ����Na2CO3��Һ����Ca2������NaOH��Һ����Mg2������BaCl2��Һ����SO![]() ����ؼ��ǰ�Na2CO3��Һ����BaCl2��Һ֮��ʹ�����������Գ�ȥ������BaCl2�����Լ����Լ��ĺ���˳��ΪC D��
����ؼ��ǰ�Na2CO3��Һ����BaCl2��Һ֮��ʹ�����������Գ�ȥ������BaCl2�����Լ����Լ��ĺ���˳��ΪC D��
��5�����Ƶõ� NaOH�������� 240 mL0.2mol/L NaOH ��Һ����Ϊû��240 mL��������ƿ������ѡ��250 mL��������ƿ��
������ʱ�����ʹ�õIJ��������н�ͷ�ιܡ�250mL����ƿ����Ͳ���ձ��������� ��
����Ϊ�������������������ձ���ʵ������Ϊ����������ȥ������������27.4g��
��.A.û��ϴ���ձ��Ͳ����������ʼ��٣�Ũ��ƫ�ͣ�B.����ƿ�����������������ˮ����Ӱ�죻C.����ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ�ߣ�D.����ʱ���ӿ̶��ߣ���Һ���ƫ��Ũ��ƫ�ͣ�E.���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ���Һ���ƫ��Ũ��ƫ�͡����Զ������Ƶ�NaOH��Һ��Ũ��ƫ�ߵIJ�����C��
��ͼ����ʾ����100mL������ƿ������û��ʹ�ò����������Դ�����δ�ò�����������δѡ��250mL����ƿ��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�