��Ŀ����
��ҵ�ϳ���Ư�۸��ᷴӦ�ų�����������������x%����ʾƯ�۵����ӡ�Ư�����ᷴӦΪ��Ca(ClO)2+CaCl2+2H2SO4=2CaSO4+2H2O+2Cl2������֪I2��Na2S2O3��Һ��ӦΪI2+2Na2S2O3=Na2S4O6+2NaI��
��Ϊ�˲ⶨһƿƯ�۵�x%������������ʵ�顣��ȡƯ����Ʒ2.00g����ˮ��ĥ��ת��250mL����ƿ�ڣ���ˮϡ�����̶ȡ�ҡ�Ⱥ�ȡ��25.0mL�����������KI��Һ������ϡH2SO4�����á���Ư�۷ų���������KI��ȫ��Ӧ����0.100mol/L Na2S2O3��Һ20.0mL�������������ݼ����Ư�۵�x%��
��Ϊ�˲ⶨһƿƯ�۵�x%������������ʵ�顣��ȡƯ����Ʒ2.00g����ˮ��ĥ��ת��250mL����ƿ�ڣ���ˮϡ�����̶ȡ�ҡ�Ⱥ�ȡ��25.0mL�����������KI��Һ������ϡH2SO4�����á���Ư�۷ų���������KI��ȫ��Ӧ����0.100mol/L Na2S2O3��Һ20.0mL�������������ݼ����Ư�۵�x%��
35.5%
��Ca(ClO)2+CaCl2+2H2SO4=2CaSO4+2H2O+2Cl2����Cl2+2KI=2KCl+I2
��I2+2Na2S2O3=Na2S4O6+2NaI�ɵ�
Cl2��2Na2S2O3
1 2
n(Cl2) 0.100mol/L��0.02L��10
��n(Cl2)= 1��10-2 mol������m(Cl2)= 1��10-2 mol��71 g��mol-1="0.71" g
x%=0.71g/2.00g=35.5%

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
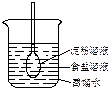
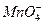 ����ԭ��Mn2+����д���ø�����ظ�Ũ��������������ȡ�����Ļ�ѧ��Ӧ����ʽ��____________________________��
����ԭ��Mn2+����д���ø�����ظ�Ũ��������������ȡ�����Ļ�ѧ��Ӧ����ʽ��____________________________��