��Ŀ����
��1��3.01��1022��OH-�����ʵ���Ϊ______����Ϊ______����ЩOH-��______mol NH3��������ȣ���______g Na+���е���������ͬ��
��2����4.6g�������ڿ����г��ȼ�յõ�����ɫ��ĩ���÷�ĩ��ˮ��Ӧ�ܹ��ų���״���µ�����______L��ʵ���������Һ��Na+�����ʵ���Ϊ______��
��3��ͬ��ͬѹͬ����İ�����NH3�������⣨H2S����������ʵ���֮��Ϊ______��ԭ�Ӹ���֮��______��
�⣺��1��n��OH-��=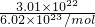 =0.05mol��m��OH-��=0.05mol��17g/mol=0.85g��
=0.05mol��m��OH-��=0.05mol��17g/mol=0.85g��
n��NH3��=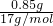 =0.05mol��
=0.05mol��
m��Na+��=0.05mol/L��23g/mol=1.15g��
�ʴ�Ϊ��0.05 mol��0.85g��0.05��1.15��
��2��n��Na��=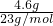 =0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
=0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
���ɵ����������Ϊv��O2��=0.05mol��22.4L/mol=1.12L��
ʵ���������Һ��Na+�����ʵ���Ϊ0.2mol��
�ʴ�Ϊ��1.12��0.2mol��
��3��ͬ��ͬѹʱ�����Vm��ͬ����n=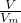 =
=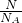 ��֪����������ʵ���֮��Ϊ1��1����ԭ�Ӹ���֮��4��3��
��֪����������ʵ���֮��Ϊ1��1����ԭ�Ӹ���֮��4��3��
�ʴ�Ϊ��1��1��4��3��
��������1������n=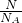 =
= ���㣻
���㣻
��2��n��Na��=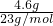 =0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2������Ϸ���ʽ���㣻
=0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2������Ϸ���ʽ���㣻
��3��ͬ��ͬѹʱ�����Vm��ͬ������n=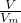 =
=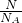 ������Ĺ��ɼ��㣮
������Ĺ��ɼ��㣮
���������⿼�����ʵ�������ؼ��㣬��Ŀ�ѶȲ���ע����ؼ��㹫ʽ�����ã�
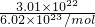 =0.05mol��m��OH-��=0.05mol��17g/mol=0.85g��
=0.05mol��m��OH-��=0.05mol��17g/mol=0.85g��n��NH3��=
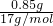 =0.05mol��
=0.05mol��m��Na+��=0.05mol/L��23g/mol=1.15g��
�ʴ�Ϊ��0.05 mol��0.85g��0.05��1.15��
��2��n��Na��=
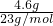 =0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
=0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2�������ɵ����������Ϊv��O2��=0.05mol��22.4L/mol=1.12L��
ʵ���������Һ��Na+�����ʵ���Ϊ0.2mol��
�ʴ�Ϊ��1.12��0.2mol��
��3��ͬ��ͬѹʱ�����Vm��ͬ����n=
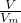 =
=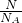 ��֪����������ʵ���֮��Ϊ1��1����ԭ�Ӹ���֮��4��3��
��֪����������ʵ���֮��Ϊ1��1����ԭ�Ӹ���֮��4��3���ʴ�Ϊ��1��1��4��3��
��������1������n=
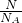 =
= ���㣻
���㣻��2��n��Na��=
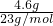 =0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2������Ϸ���ʽ���㣻
=0.2mol������ɫ��ĩ��ˮ��Ӧ����ʽΪ2Na2O2+2H2O=4NaOH+O2������Ϸ���ʽ���㣻��3��ͬ��ͬѹʱ�����Vm��ͬ������n=
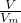 =
=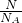 ������Ĺ��ɼ��㣮
������Ĺ��ɼ��㣮���������⿼�����ʵ�������ؼ��㣬��Ŀ�ѶȲ���ע����ؼ��㹫ʽ�����ã�

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ
����˵����ȷ����
| A��0.25 mol CO2 | B��1 mol����6.02��10 23������ |
| C��1 mol �� | D��0.5 mol H2 ���� 3.01��10 23 ����ԭ�� |