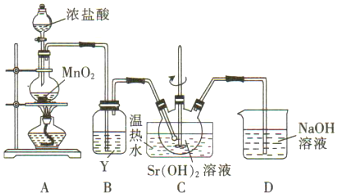
��Ŀ����
����Ŀ���Զ�����̼������Ϊԭ����ȡ�Ҵ��ķ�ӦΪ2CO2(g)��6H2(g)![]() CH3CH2OH(g)��3H2O(g) ��H��0��ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1:3Ͷ�ϣ���ͬ�¶���ƽ����ϵ�и����ʵ����ʵ����ٷ���(y%)���¶ȱ仯��ͼ��ʾ������˵����ȷ����
CH3CH2OH(g)��3H2O(g) ��H��0��ijѹǿ�µ��ܱ������У���CO2��H2�����ʵ�����Ϊ1:3Ͷ�ϣ���ͬ�¶���ƽ����ϵ�и����ʵ����ʵ����ٷ���(y%)���¶ȱ仯��ͼ��ʾ������˵����ȷ����
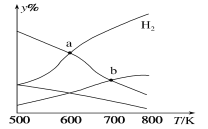
A.a���ƽ�ⳣ��С��b��
B.b�㣬v��(CO2)��v��(H2O)
C.a�㣬H2��H2O���ʵ������
D.���������㶨���������H2��v(CO2)����
���𰸡�C
��������
A. ��Ϊƽ�ⳣ�������¶��й�,�÷�ӦΪ���ȷ�Ӧ,�����¶�ƽ�����淴Ӧ�ƶ�,�����¶�Խ��,KԽ��,����Ka��Kb����A����
B. b��Ϊ���¶��µ�ƽ��״̬�����ݷ���ʽ��֪v��(CO2)��v��(H2O)����B����
C. ����ͼ�������Ϸ���ʽ��֪a��ΪH2��H2O���ʵ����Ľ��㣬������ȣ���C��ȷ��
D. ���������㶨,�������H2������Ӧ���Ũ��ƽ�������ƶ�������v(CO2)Ҳ�ı䣬��D����
��ѡ��C��

��ϰ��ϵ�д�
�����Ŀ