��Ŀ����
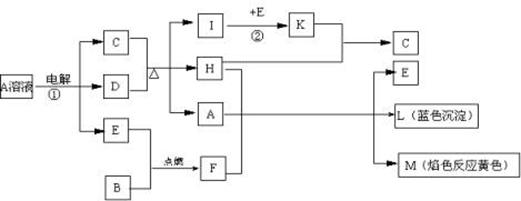
(14��)�����ֽ�������A��B��C������A����ɫ��ӦΪ��ɫ��B��C�dz������������ֽ�������A��B��C��������ס��ҡ���������D��E��F��G��H֮�䷢������ת����ϵ��ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û�б������
�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽ��
A_____________��H ______________�� G___________����____________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ��________________________________����Ӧ��_________________________________��
��3�� ��Ӧ���е���ת�Ƶ���ĿΪ6.02��1023��ʱ���ĵ������ҵ����ʵ���Ϊ mol
��1��Na����2�֣� Fe(OH)3����2�֣� FeCl3����2�֣� Cl2��2�֣�
��2��Fe+2HCl=FeCl2+H2 �� ��2�֣���2FeCl2+Cl2="=2" FeCl3 ��2�֣�����3��0.5mol��2�֣�
����������������������֪����A��Na;����B��Al������C��Fe���������H2����������Cl2;�������HCl����ҺD��NaOH ; ��ҺE�������ҺF��FeCl2����ҺG��FeCl3������H��Fe(OH)3����1���������ʵĻ�ѧʽ�ֱ��ǣ�A��Na��H��Fe(OH)3��G��FeCl3������Cl2; ��2����Ӧ�ٻ�ѧ����ʽ�ǣ�Fe+2HCl=FeCl2+H2������Ӧ�ڻ�ѧ����ʽ��2FeCl2+Cl2="=2" FeCl3 ����3���ڷ�Ӧ2FeCl2+Cl2="=2" FeCl3 ��֪ÿ����1mol������������������ԭ��Ӧת�Ƶ���2NA,������ת�Ƶ���ĿΪ6.02��1023��ʱ���ĵ������ҵ����ʵ���Ϊ0.5mol��
���㣺����Ԫ�ؼ���������ƶϡ����ʵĻ�ѧʽ����д����ѧ����ʽ����д��������ԭ��Ӧ�ļ����֪ʶ��

 ��У����ϵ�д�
��У����ϵ�д���һС������Ʒ�����������ȣ�ʵ������������ȷ���ǣ�
������ȼ��֮ǰ���ۻ� ����ȼ�յĻ������ɫ
����ȼ�պ�õ���ɫ���� ����ȼ�յĻ���ʻ�ɫ
����ȼ�պ�������Ϊ����ɫ����
������ȷ����
| A��ֻ�Т� | B��ֻ�Т� | C���٢ܢ� | D���ڢ� |
�ú�����þ�۵�������ȡ��������������������������������ǡ���������
�ټ������ܽ� �ڼ��ռ���Һ�ܽ� �۹��� ��ͨ�����CO2����Al(OH)3����
�ݼ�����������Al(OH)3���� ��������ռ���Һ
| A���٢ޢݢ� | B���ڢۢܢ� | C���ڢۢݢ� | D���٢ۢݢ� |
�������ƣ�Na2FeO4�����к�ǿ�������ԣ���һ�����͵���ɫ���Ⱦ�ˮ��������������ز��ϡ��Դ�FeO������CuO��Al2O3��SiO2�����ʣ��Ʊ��������Ƶ������������£�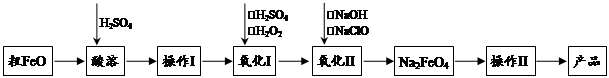
��֪��NaClO���ȶ��������ֽ⡣�ش��������⣺
��1���������ƣ�Na2FeO4������Ԫ�صĻ��ϼ�Ϊ ��
��FeO���ܹ�����ͨ�����ˮ��������Ŀ������������������ ��������
��2��������Ŀ���ǵõ��ߴ���FeSO4��Һ������������Һ������μ�KSCN��Һ����Һ��ΪѪ��ɫ���ɴ��Ʋ�������Ӧ�����ӷ���ʽΪ������ ����������
��3������������Ҫ��Ũ��NaClO��Һ������Cl2��NaOH��Һ��Ӧ�Ʊ���
��Cl2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
���ڲ�ͬ�¶��½��и÷�Ӧ����Ӧ��ͬһ��ʱ��������NaClOŨ�����£�
| �¶�/0C | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| NaClOŨ��/mol��L-1 | 4.6 | 5.2 | 5.4 | 5.5 | 4.5 | 3.5 | 2 |
_______________________________________________________________________________________��
����ʯ��һ�ָ�þ�����ο�����ܳƣ�������ɫ������������Ƥ������������ʯ���Կ�����MgO��FeO��Fe2O3��Al2O3��SiO2��ɡ���ҵ��������ʯ��ȡ��ʽ̼��þ��Ʒ���������£�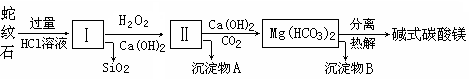
��1������ʯ�������ܽ����Һ�����Mg2����Al3+�⣬�����еĽ���������________��
��2������м���H2O2�������� ���й����ӷ���ʽ ������Ca(OH)2ʱ�� ��Ҫ������ҺpH��7��8֮��(�й��������������pH���±�)��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
��3��������A����Ϊ��ȡ��ɫ���ϵ�ԭ�ϣ����������A�м��� ��Һ��Ȼ����ˡ�ϴ�ӡ� _________����дʵ���������)�����ɻ�ú�ɫ���ϣ�ʵ�ַ�����ۺ����á�
��4������Ʒ�Ļ�ѧʽ��aMgCO3��bMg(OH)2��cH2O��ʾ���ֳ�ȡ18.2 g��Ʒ��ʹ֮��ȫ�ֽ⣬�ռ���3.36L CO2����״���£���8.0 g MgO��ͨ������ȷ����Ʒ�Ļ�ѧʽ�У�a��________��b��________��c��________��
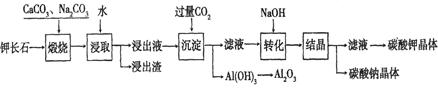
 Al(OH)3+ OH��������ȡ��ʱӦ������Һ��________��(��ᡱ���������ȡ��ʱ���Ͻ����Ŀ����_____ ____��
Al(OH)3+ OH��������ȡ��ʱӦ������Һ��________��(��ᡱ���������ȡ��ʱ���Ͻ����Ŀ����_____ ____��