��Ŀ����
����Ŀ����ͭ��Cu3P2��������������ͭ������ͭ�Ǻ�����������ͭ�Ͻ���Ҫ������ĥ����͵���ԭ����
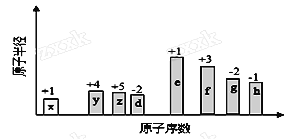
��1����̬ͭԭ�ӵĵ����Ų�ʽΪ______���۵����гɶԵ�������____����
��2����ͭ��ˮ���ò����ж������⣨PH3����
��PH3�����е�����ԭ�ӵ��ӻ���ʽ��_________��
��P��Nͬ���壬������������Ӧˮ��������ԣ�HNO3___H3PO4������>������<�������ӽṹ�ĽǶ�˵�����ɣ�__________________________��
��3������ͭ�е���������Ԫ�ص縺�ԵĴ�СΪSn___P������>����<������=������
��4��ij����ͭ�����ṹ��ͼ��ʾ��
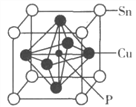
�����仯ѧʽΪ________��
�ڸþ����о���Cuԭ�������Snԭ����______������ЩSnԭ�������ֵĹ���Ϊ_________��
���������ܶ�Ϊ8.82g��cm��3�������Cuԭ�Ӻ˼��Ϊ____pm���ú�NA�Ĵ���ʽ��ʾ����
���𰸡� 1s22s22p63s23p63d104s1��[Ar]3d104s1 10 sp3 > ��ΪHNO3���ӽṹ�к���2����������ԭ������H3PO4�ж�1�� < SnCu3P 4 ƽ�������� 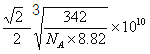
�������������������1�����ݺ�������Ų����ɽ��
��2�������ݼ۲���ӶԻ������۷�����
�ڸ���Ӱ�캬�������Ե����ط����жϣ�
��3�����ݵ縺�Ա仯���ɽ��
��4�����ݾ����ṹ��Ͼ�̯���������ж�����㡣
��������1��ͭ��ԭ��������29����̬ͭԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1����˼۵����гɶԵ�������10����
��2����PH3������Pԭ�Ӻ��еŶԵ��Ӷ�����![]() �����۲���Ӷ���Ϊ4����������ԭ�ӵ��ӻ���ʽ�� sp3��
�����۲���Ӷ���Ϊ4����������ԭ�ӵ��ӻ���ʽ�� sp3��
������HNO3���ӽṹ�к���2����������ԭ�ӣ���H3PO4�ж�1��������������������Ӧˮ�����������HNO3>H3PO4��
��3���ǽ�����Խǿ���縺��Խ������������Ԫ�ص縺�ԵĴ�СΪSn>P��
��4���ٸ��ݾ����ṹ��֪���е�Snԭ�Ӹ�����8��1/8��1��Cuԭ�Ӹ�����6��1/2��3��Pλ�����ģ�����1�������仯ѧʽΪSnCu3P��
�ڸþ����о���Cuԭ�������Snԭ����4����λ�����4�������ϣ������ЩSnԭ�������ֵĹ���Ϊƽ�������Ρ�
�۸��ݾ����ṹ��֪�����Cuԭ�Ӻ˼��Ϊ��Խ��ߵ�һ�㣬�����ı߳���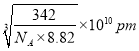 �����������Cuԭ�Ӻ˼��Ϊ
�����������Cuԭ�Ӻ˼��Ϊ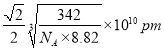 ��
��

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�����Ŀ��ijС��ͬѧΪ̽�� H2O2�� H2SO3�� Br2 ������ǿ�����������ʵ�飨�г���������ȥ��װ�õ��������Ѽ��飩��

ʵ���¼���£�
ʵ����� | ʵ������ | |
�� | ���� a���μ���ˮ���رջ��� a | _____________________________________________ |
�� | �����ȿ���һ��ʱ���ֹͣ | A����Һ��ɫ���Ա�dz��B�������ݣ�����������ɫ�������������ϲ���ҺΪ��ɫ |
�� | ����b����μ���H2O2��Һ | ��ʼʱ��Һ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɳȺ�ɫ�� |
���������գ�
��1���ڽ��в�����ʱ��A�е�ʵ��������___________���йط�Ӧ�����ӷ���ʽ��___________��
��2�������ڴ����ȿ�����Ŀ����____________��B �в�����ɫ�����Ļ�ѧʽ��___________��
��3��װ��C��������____________________��
��4��������ʵ���֪���ڴ�ʵ�������£�H2O2��H2SO3��Br2������ǿ��˳��Ϊ________________��
��5�������ۿ�ʼʱ��ɫ�����Ա仯����ԭ���ǣ�д��һ�����ɣ���___________________��
����Ŀ�������ܿ�����ָʾ���ʹ������Ʊ�����ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��A12O3��MnO��MgO��CaO��SiO2�ȣ���ȡCoC2O4��2H2O�����������£�

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�����������£�ClO3-��������Co2+��ClO3-ת��ΪCl-��
�۲���������������������ʽ����ʱ��Һ��pH������
������ | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
��ȫ������pH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
��1�����������м���Na2SO3����ҪĿ����________��
��2�������Һ�м���NaClO3�����ӷ�Ӧ����ʽ��_________��
��3����֪��������NH3��H2O![]() NH4+��OH- Kb��1.8��10-5
NH4+��OH- Kb��1.8��10-5
H2C2O4![]() H+��HC2O4- Ka1��5.4��10-2
H+��HC2O4- Ka1��5.4��10-2
HC2O4-![]() H��C2O42- Ka2��5.4��10-5
H��C2O42- Ka2��5.4��10-5
�������������(NH4)2C2O4��Һ��pH______7�������������=������
��4������(NH4)2C2O4 ��Һ���������壬�ٹ��ˡ�ϴ�ӣ�ϴ��ʱ��ѡ�õ��Լ��У�________��
A������ˮ B������ˮ C�����͵�(NH4)2C2O4��Һ D��ϡ����
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ����ͼ1����ȡ����������________����ʹ�õ�����pH��Χ��________��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5
��6��CoC2O4��2H2O�ȷֽ������仯������ͼ2��ʾ������600����ǰ�Ǹ����������ȣ�600 ���Ժ����ڿ����м��ȡ�A��B��C��Ϊ�����C����ʾ����Ļ�ѧʽ��________��