��Ŀ����
����Ŀ��A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ����������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ����ʾ�� 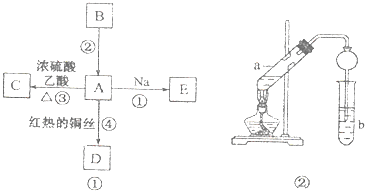
��1��A�й����ŵ�����Ϊ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��Ӧ������Ӧ����
��3��ʵ�������÷�Ӧ����ȡC��������ͼ��װ�ã� ��a�Թ��е���Ҫ��ѧ��Ӧ�ķ���ʽΪ�� ��
����ʵ�������θ���ܳ������������⣬��һ����Ҫ������ ��
���Թ�b�й۲쵽�������� ��
���𰸡�
��1���ǻ�
��2��2CH3CH2OH+2Na��2CH3CH2ONa+H2����CH3CH2OH+O2 ![]() 2CH3CHO+2H2O
2CH3CHO+2H2O
��3��CH3CH2OH+CH3COOH ![]() CH3COOCH2CH3+H2O����������Һ��ֲ�
CH3COOCH2CH3+H2O����������Һ��ֲ�
���������⣺B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ����BΪCH2=CH2 �� A�ǻ�ѧʵ����������л����������ˮ����������ζ��Bת���õ�A��Ӧ����ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���������������DΪCH3CHO���Ҵ������ᷢ��������Ӧ����CΪCH3COOCH2CH3 �� �Ҵ����Ʒ�Ӧ����EΪCH3CH2ONa����1��������������֪��AΪCH3CH2OH�����й�����Ϊ���ǻ������Դ��ǣ��ǻ�����2����Ӧ�����Ҵ����Ʒ�Ӧ�����Ҵ�������������Ӧ����ʽΪ��2CH3CH2OH+2Na��2CH3CH2ONa+H2������Ӧ�����Ҵ��Ĵ���������Ӧ�Ļ�ѧ����ʽΪCH3CH2OH+O2 ![]() 2CH3CHO+2H2O�����Դ��ǣ�CH3CH2OH+2Na��2CH3CH2ONa+H2����CH3CH2OH+O2
2CH3CHO+2H2O�����Դ��ǣ�CH3CH2OH+2Na��2CH3CH2ONa+H2����CH3CH2OH+O2 ![]() 2CH3CHO+2H2O����3����a�Թ�Ϊ�Ҵ���������Ũ���ᡢ���������·���������Ӧ��������������ˮ����Ӧ����ʽΪ��CH3CH2OH+CH3COOH
2CH3CHO+2H2O����3����a�Թ�Ϊ�Ҵ���������Ũ���ᡢ���������·���������Ӧ��������������ˮ����Ӧ����ʽΪ��CH3CH2OH+CH3COOH ![]() CH3COOCH2CH3+H2O�����Դ��ǣ�CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O�����Դ��ǣ�CH3CH2OH+CH3COOH ![]() CH3COOCH2CH3+H2O������ʵ�������θ���ܳ����������ã�ͬʱ��ֹ���������Դ��ǣ���������������������������к������ᡢ�Ҵ���ͨ���ñ���̼������Һ����ȥ�ӷ�������������Ҵ�����������������ˮ��Һ��ֲ㣬���Դ��ǣ�Һ��ֲ㣮
CH3COOCH2CH3+H2O������ʵ�������θ���ܳ����������ã�ͬʱ��ֹ���������Դ��ǣ���������������������������к������ᡢ�Ҵ���ͨ���ñ���̼������Һ����ȥ�ӷ�������������Ҵ�����������������ˮ��Һ��ֲ㣬���Դ��ǣ�Һ��ֲ㣮





