��Ŀ����
ͼ8-3��ij����X�������о������ʵ�װ�á�
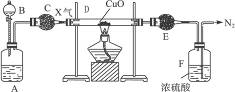
ͼ8-3
A��ʢ���ܵİ�ɫ���壬B��ʢ����ɫ�ӷ���Һ�壬C��E��ʢ�и������A��B����������ʱ����ɫ����X���ɣ�����ͼ��һϵ��װ����ĩ�˵õ�N2����E�ܵ��������ӡ�
��1��д��ʢ���Լ������ƣ�
A.____________��B.____________��C.____________��E.____________��
��2��A��B��������������X����Ҫԭ���ǣ�________________________��
��3��D�з�Ӧ�Ļ�ѧ����ʽ_________________________________________��
��4��F�з�Ӧ�Ļ�ѧ����ʽ_________________________________________��
��5����D�з�Ӧ˵������X����____________������ԡ������ԡ��������ԡ���ԭ�ԡ�����

ͼ8-3
A��ʢ���ܵİ�ɫ���壬B��ʢ����ɫ�ӷ���Һ�壬C��E��ʢ�и������A��B����������ʱ����ɫ����X���ɣ�����ͼ��һϵ��װ����ĩ�˵õ�N2����E�ܵ��������ӡ�
��1��д��ʢ���Լ������ƣ�
A.____________��B.____________��C.____________��E.____________��
��2��A��B��������������X����Ҫԭ���ǣ�________________________��
��3��D�з�Ӧ�Ļ�ѧ����ʽ_________________________________________��
��4��F�з�Ӧ�Ļ�ѧ����ʽ_________________________________________��
��5����D�з�Ӧ˵������X����____________������ԡ������ԡ��������ԡ���ԭ�ԡ�����
��1����ʯ�� Ũ��ˮ ��ʯ�� ��ʯ��
(2)CaO��ˮ����������ʯ�ң�����ˮ���ų������ȣ�ʹŨ��ˮ�е�NH3�ݳ�
��3��3CuO+2NH3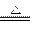 N2+3Cu+3H2O
N2+3Cu+3H2O
(4)2NH3+H2SO4====(NH4)2SO4
(5)��ԭ��
(2)CaO��ˮ����������ʯ�ң�����ˮ���ų������ȣ�ʹŨ��ˮ�е�NH3�ݳ�
��3��3CuO+2NH3
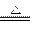 N2+3Cu+3H2O
N2+3Cu+3H2O(4)2NH3+H2SO4====(NH4)2SO4
(5)��ԭ��
��ĩ�˵�N2���ƿ�֪X�к�NԪ�ء���E���ؿ��Ƶ�D�з�Ӧ��ˮ���ɡ�N2��H2O����D�з�Ӧ�����������ж�D��CuO�뺬N��HԪ�ص�����X���ã�����XΪNH3��A��B������NH3��BӦʢ��Ũ��ˮ��A��Ӧʢ����NaOH��CaO������������ܣ���֪����CaO�����ȫ������֤��

��ϰ��ϵ�д�
 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ
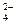 ��NH
��NH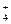 ��Ba2+��H2PO
��Ba2+��H2PO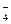 ��NO
��NO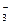 �е�һ�ֻ��֣��ֱ�ȡ������Һ��������ʵ�飺�ٴ���Һ����NaOH��Һ�г������������Ȳ���һ��ʹʪ���ɫʯ����ֽ���������塣����ȡԭ��ҺŨ�������Ũ���ᡢͭƬ�����ȣ���������ɫ���塣�ɴ˿�֪����Һ��һ������_______���ӣ�һ��������_______���ӡ�
�е�һ�ֻ��֣��ֱ�ȡ������Һ��������ʵ�飺�ٴ���Һ����NaOH��Һ�г������������Ȳ���һ��ʹʪ���ɫʯ����ֽ���������塣����ȡԭ��ҺŨ�������Ũ���ᡢͭƬ�����ȣ���������ɫ���塣�ɴ˿�֪����Һ��һ������_______���ӣ�һ��������_______���ӡ�