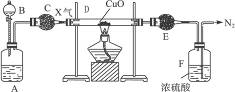
��Ŀ����
��1����̨�ϵ�Ļ���벼��������ŨNH4Cl��Һ�����Ƴɵģ����Է�����ԭ����_____________________________��
��2����֪NH3���H+�������Ƚ��Ag+������ǿ��Ҫʹ��Ag(NH3)2��+ת��Ϊ���ɵ�Ag+��Ӧ������Լ���_______��ʵ��ת�������ӷ���ʽΪ______________________________________��
��3������������NOx��NH3��Ӧ����N2��H2O�����ڱ�״����1.5 L NOx��2 L NH3ǡ����ȫ���ã�д����Ӧ�Ļ�ѧ����ʽ��____________________________��NOx��xֵΪ___________��
��2����֪NH3���H+�������Ƚ��Ag+������ǿ��Ҫʹ��Ag(NH3)2��+ת��Ϊ���ɵ�Ag+��Ӧ������Լ���_______��ʵ��ת�������ӷ���ʽΪ______________________________________��
��3������������NOx��NH3��Ӧ����N2��H2O�����ڱ�״����1.5 L NOx��2 L NH3ǡ����ȫ���ã�д����Ӧ�Ļ�ѧ����ʽ��____________________________��NOx��xֵΪ___________��
��1��NH4Cl���ȷֽ�ʱ����������������ϵ�¶ȣ�ͬʱ�ֽ������NH3��HCl�ܸ�������
��2��HNO3 ��Ag(NH3)2��++2H+====Ag++2NH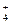
(3)6NOx+4xNH3====(3+2x)N2+6xH2O 2
��2��HNO3 ��Ag(NH3)2��++2H+====Ag++2NH
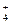
(3)6NOx+4xNH3====(3+2x)N2+6xH2O 2
��1����NH4Cl���ȷֽ����NH3��HCl���ǡ� ��2���������ϢӦ��H+,��������HCl��H2SO4,��ΪCl-��SO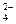 ����Ag+��ϡ���NOx��NH3��Ӧ���������x��ֵ��1.5��2=6��4x�����x=2��
����Ag+��ϡ���NOx��NH3��Ӧ���������x��ֵ��1.5��2=6��4x�����x=2��
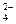 ����Ag+��ϡ���NOx��NH3��Ӧ���������x��ֵ��1.5��2=6��4x�����x=2��
����Ag+��ϡ���NOx��NH3��Ӧ���������x��ֵ��1.5��2=6��4x�����x=2��
��ϰ��ϵ�д�
�����Ŀ