��Ŀ����
����Ŀ��ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA��E���������������Ҫ����ա�

(1)д���������ƣ�C__________��E_________��
(2)����ʵ��������õ�����E����___________������ĸ����
a.����ˮ��CCl4�Ļ����
b.����ˮ�;ƾ��Ļ����
c.����ˮ����ɳ�Ļ����
d.����NaCl��Һ�е�NaCl��ˮ
(3)����A��E��ʹ��ǰ�������Ƿ�©ˮ����_________��
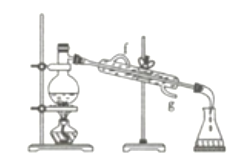
(4)��������ͼװ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������____________������ǰӦ����Բ����ƿ�м��뼸����ʯ��Ŀ����_________________________�������ܵĽ�ˮ����________������f������g������
���𰸡�����ͨ��©�� ��Һ©�� a DE �¶ȼ� ��ֹ���� g
��������
(1)����������״д�����ƣ�
(2)����GΪ��Һ©���������ڷ�Һ��
(3)����������ʹ��Ŀ���ж��ĸ���Ҫ����Ƿ�©Һ��
(4)����������Һ������Ҫ���¶ȼƲ�������¶ȣ�Ҫ��ֹ��������ķ�������������ԭ�����¡�
(1)����������״C��©����EΪ��Һ©����
(2)����GΪ��Һ©���������ڷ��뻥�����ݵ�����Һ�����ʣ�
a.ˮ��CC14�ǻ������ݵ�����Һ�����ʣ����÷�Һ©�����룬a��ȷ��
b.�ƾ���ˮ���ܵ�Һ�����ʣ������÷�Һ���룬b����
c.��ɳ��������ˮ�Ĺ������ʣ�Ӧ�ù��˷������룬c����
d.ʳ��ˮ�е�����NaCl���ܼ�ˮ�������ܼ������룬d����
�ʺ���ѡ����a��
(3)A����Ͳ��������ȡһ�������Һ�����ʣ�����Ҫ����Ƿ�©Һ��A����
B.���ձ����������ȡ�������Һ��ϡ�͵ȣ�����Ҫ����Ƿ�©Һ��B����
C.��װ����©�����������˻���С��������ת����Һ������Ҫ����Ƿ�©Һ��C����
D.Ϊ����ƿ��ֻ���ڳ�����ʹ�ã���������һ�����һ��Ũ�ȵ���Һ����ʹ��ʱҪ����Һҡ�ȣ����ʹ��ǰҪ����Ƿ�©ˮ��D��ȷ��
E.Ϊ��Һ©�������ڷ��뻥�����ݵ�����Һ�����ʣ�Ϊ��ֹ�����Һ�������ٻ�ϣ�ʹ��ǰҪ����Ƿ�©ˮ��E��ȷ��
�ʺ���ѡ����DE��
(4)���Ȼ�̼�;ƾ��ǻ��ܵ�Һ��������ڶ��߷е����ϴɲ����������룬����ͼʾ��֪����ȱ�ٵ��������¶ȼƣ�Һ���������ʱ��Ϊ��ֹҺ�屩�У�Ҫ�������Ƭ�������Ȳ�������ֽ��£�Ϊ��������Ч����ʹ�������г�����ˮ��Ҫ��������ԭ����������ˮ�����g��