��Ŀ����
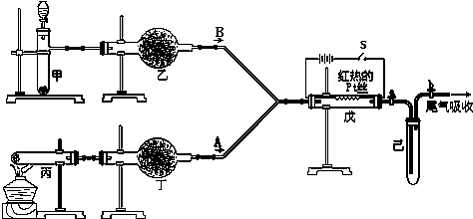
ij����С���������ͼ��ʾ��ʵ��װ�ã�������������ʵ�飮ͼ�м�ͷ��ʾ��������A��ʾһ�ִ�������������壬B����һ�����壬��Ӧ����һ��ʱ���װ�ü����к���ɫ�������ɣ�
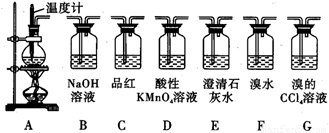
ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����Ũ���ᡢ����ˮ��
����ͼ��װ�úͷ�Ӧ����ش�
��1����ַ�Ӧ�������������ʣ�࣬������Ӧ�Ļ�ѧ����ʽΪ______��
��2�����еĸ����Ӧѡ______����ѡ��һ�ָ������������______��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��4���۲쵽��Ӧ��ʼ��Ͽ����S����˿�ܼ������ֺ��ȣ����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ______���˷�Ӧ�ǣ����ȡ����ȣ�______��Ӧ��
��5����װ�õ����������ã��ס����о�����������������Լ�ѡ����ȷ������������������δ�۲쵽����ɫ�����ܵ�ԭ����______��

ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����Ũ���ᡢ����ˮ��
����ͼ��װ�úͷ�Ӧ����ش�
��1����ַ�Ӧ�������������ʣ�࣬������Ӧ�Ļ�ѧ����ʽΪ______��
��2�����еĸ����Ӧѡ______����ѡ��һ�ָ������������______��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ______��
��4���۲쵽��Ӧ��ʼ��Ͽ����S����˿�ܼ������ֺ��ȣ����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ______���˷�Ӧ�ǣ����ȡ����ȣ�______��Ӧ��
��5����װ�õ����������ã��ס����о�����������������Լ�ѡ����ȷ������������������δ�۲쵽����ɫ�����ܵ�ԭ����______��
��1�����ݳ�ַ�Ӧ�������������ʣ�ֻ࣬��̼����立����������ʴ�Ϊ��NH4HCO3
NH3��+CO2��+H2O��
��2��װ�ñ���ȡ�������ܸ��ﰱ����ֻ�������Ը��������Ը�����������ܺ���ˮ�Ȼ��Ʒ�Ӧ������λ�������ӦΪ��CaCl2+8NH3�TCaCl2?8NH3���ʲ��������Ը����CaCl2����NH3���壮
�ʴ�Ϊ����ʯ�ң���Ϊ��ˮCaCl2�����백��϶����õ�������
��3����Ŀ���ṩ��ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����Ũ���ᡢ����ˮ��װ�ü�������������ȡ����������ֻ��ѡNa2O2���ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2������4�������������Ͱ�����ϵ�װ�ã���������Ҫ��Ӧ�ǰ����Ĵ�����������һ���������Ͽ����S����˿�ܼ������ֺ��ȣ�˵���÷�ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O ���ȣ�
��5����������һ������Ϊ��ɫ������δ�۲쵽����ɫ�Ķ���������˵������δ���뼺�У�װ�õ����������ã��ס����о������������ֻ��˵�����в���������̫�٣��ʴ�Ϊ�����в���������̫�٣�
| ||
��2��װ�ñ���ȡ�������ܸ��ﰱ����ֻ�������Ը��������Ը�����������ܺ���ˮ�Ȼ��Ʒ�Ӧ������λ�������ӦΪ��CaCl2+8NH3�TCaCl2?8NH3���ʲ��������Ը����CaCl2����NH3���壮
�ʴ�Ϊ����ʯ�ң���Ϊ��ˮCaCl2�����백��϶����õ�������
��3����Ŀ���ṩ��ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����Ũ���ᡢ����ˮ��װ�ü�������������ȡ����������ֻ��ѡNa2O2���ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2������4�������������Ͱ�����ϵ�װ�ã���������Ҫ��Ӧ�ǰ����Ĵ�����������һ���������Ͽ����S����˿�ܼ������ֺ��ȣ�˵���÷�ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ��4NH3+5O2
| ||
| ���� |
��5����������һ������Ϊ��ɫ������δ�۲쵽����ɫ�Ķ���������˵������δ���뼺�У�װ�õ����������ã��ס����о������������ֻ��˵�����в���������̫�٣��ʴ�Ϊ�����в���������̫�٣�

��ϰ��ϵ�д�
�����Ŀ