��Ŀ����
������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB=(NH?3ͨ�����·�Ӧ�Ƶã�3CH4��2?HB=NH)3��6H2O=3CO2��6H3BNH3���Ժϳ�A��Ӧ����ʽ�������в���ȷ����(����)
| A����Ӧǰ��̼ԭ�ӵ��ӻ����Ͳ��� |
| B�����ǵĴ�С��ϵ��CO2>CH4>H2O |
| C����һ�����ܴ�С��ϵ��N>O>C>B>H |
| D��������H3BNH3�д�����λ�� |
��A
����

��ϰ��ϵ�д�
�����Ŀ
�����йؽṹ�����ʵ�˵���в���ȷ���� (�� )
| A��Ԫ�����ڱ��дӢ�B�嵽��B��ʮ�����е�Ԫ�ض��ǽ���Ԫ�� |
| B�����ԣ�NaOH > NH3��H2O ,����Ԫ�صĽ����ԣ�Na > N |
| C��ͬ���ڵڢ�A����ڢ�A���Ԫ��ԭ������֮�һ��Ϊ1 |
| D���ڢ�A��Ԫ�ش��ϵ��£����⻯����ȶ������� |
���з�Ӧ�����У�ͬʱ�����Ӽ������Թ��ۼ��ͷǼ��Թ��ۼ��Ķ��Ѻ��γɵķ�Ӧ�ǣ� ��
| A��NH4Cl=NH3��+HCl�� |
| B��NH3+CO2+H2O=NH4HCO3 |
| C��2NaOH+Cl2=NaCl+NaClO+H2O |
| D��2Na2O2+2CO2=2Na2CO3+O2 |
����˵���д�����ǣ���������
| A��SO2��SO3���Ǽ��Է��� |
| B����NH4+��[Cu��NH3��4]2���ж�������λ�� |
| C��Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խ�� |
| D��ԭ�Ӿ�����ԭ���Թ��ۼ���ϣ����м��ܴ��۵�ߡ�Ӳ�ȴ������ |
W��X��Y��Z���ֶ�����Ԫ����Ԫ�����ڱ��е����λ����ͼ��ʾ��W����̬�⻯���������ۺ����ᷴӦ�������ӻ��������˵������ȷ���ǣ���������
| W | X | |
| | Y | Z |
A��X��Y��Z������⻯���ȶ�����������Y
B��ZԪ���������Ӧˮ���������һ��ǿ��Y
C��XԪ���γɵĵ��������ӻ�ԭ������Y
D��YZ2�����и�ԭ�ӵ�����������8e���ȶ��ṹ
��֪W��X��Y��ZΪ������Ԫ�أ�W��Zͬ���壬X��Y��Zͬ���ڣ�W����̬�⻯����ȶ��Դ���Z����̬�⻯����ȶ��ԣ�X��YΪ����Ԫ�أ�X�������ӵ�������С��Y�������ӵ������ԡ�����˵����ȷ����(����)
| A��X��Y��Z��W��ԭ�Ӱ뾶���μ�С |
| B��W��X�γɵĻ�������ֻ�����Ӽ� |
| C��W����̬�⻯��ķе�һ������Z����̬�⻯��ķе� |
| D����W��Y��ԭ���������5��������γɻ�����Ļ�ѧʽһ��ΪY2W3 |
������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Wԭ�ӵ����������������ڲ��������3���������ж���ȷ���� (����)��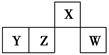
| A��ԭ�Ӱ뾶��r(W)>r(Z)>r(Y)>r(X) |
| B����YԪ�ص�����Һ�е������ԣ��е��Լ��� |
| C�������̬�⻯������ȶ��ԣ�Z>W |
| D��X����Ԫ����ɵĻ�����XH5��ˮ��Ӧ�ɲ����������� |
 ������ԭ���У����Ԫ��ԭ�ӵĻ�ѧ�������Ƶ��ǣ� ��
������ԭ���У����Ԫ��ԭ�ӵĻ�ѧ�������Ƶ��ǣ� �� 