��Ŀ����
�ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮
(1)�����°�����������ˮ����ˮ��Һ���Ե��磮
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ������__________________
�ڰ�ˮ��ˮ�������c(OH��)________10��7 mol/L(��д������������������)
�۽���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ�����Ϊ________��
(2)�������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96 L����(��״��)��ͬʱ����0.3 mol��A��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ________��
���ڱ�״���£�ÿ����1 mol��B��ת�Ƶ��ӵ����ʵ���Ϊ________mol��
(3)����ijѹǿ�㶨���ܱ������м���2 mol��N2��4 mol��H2���������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����H����92.4 kJ/mol
2NH3(g)����H����92.4 kJ/mol
�ﵽƽ��ʱ�����Ϊ��Ӧǰ������֮������
�ٴﵽƽ��ʱ��N2��ת����Ϊ________��
������������м���a mol��N2��b mol��H2��c mol��NH3����a��b��c����0������ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ���������ƽ����ͬ���ԱȽϷ�Ӧ�ų�����������________��(�����������������)
������2 mol��N2��4 mol��H2������ʼ�����ͬ�ĺ��������У��������ͬ���¶��´ﵽƽ�⣮
���ԱȽ�ƽ��ʱNH3��Ũ�ȣ���________��(�����������������)��
������
|
����(1)��NH3��H2O �����ڣ�����1�� ������c(Cl��)��c(NH4+)��c(H+)��c(OH��)����1�� ����(2)��4NH3��3F2 ������6����1�� ����(3)��50������1�� �����ڣ�����1�� �����ۣ�����1�� |

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�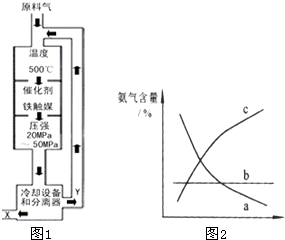 ��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�
��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�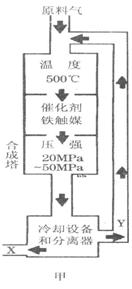
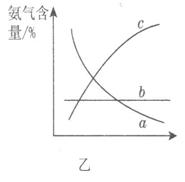
 ��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________�� ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
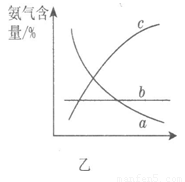
 ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��