��Ŀ����
�ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮
��1�������°�����������ˮ����ˮ��Һ���Ե��磮
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ������
�ڰ�ˮ��ˮ�������c��OH-��
�۽���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ�����Ϊ
��2���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ
���ڱ�״���£�ÿ����1mol B��ת�Ƶ��ӵ����ʵ���Ϊ
��3������ijѹǿ�㶨���ܱ������м���2mol N2��4mol H2���������·�Ӧ��
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol�ﵽƽ��ʱ�����Ϊ��Ӧǰ������֮������
�ٴﵽƽ��ʱ��N2��ת����Ϊ
������������м���a mol N2��b mol H2��c mol NH3����a��b��c����0������ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ���������ƽ����ͬ���ԱȽϷ�Ӧ�ų�����������
������2mol N2��4mol H2������ʼ�����ͬ�ĺ��������У��������ͬ���¶��´ﵽƽ�⣮
���ԱȽ�ƽ��ʱNH3��Ũ�ȣ���
��1�������°�����������ˮ����ˮ��Һ���Ե��磮
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ������
NH3+H2O?NH3?H2O?NH4++OH-
NH3+H2O?NH3?H2O?NH4++OH-
�ڰ�ˮ��ˮ�������c��OH-��
��
��
10-7mol/L����д����������������=�����۽���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ�����Ϊ
c��Cl-����c��NH4+����c��H+����c��OH-��
c��Cl-����c��NH4+����c��H+����c��OH-��
����2���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ
4NH3+3F2
NF3+3NH4F������Ҫע����ͭ��
| ||
4NH3+3F2
NF3+3NH4F������Ҫע����ͭ��
��
| ||
���ڱ�״���£�ÿ����1mol B��ת�Ƶ��ӵ����ʵ���Ϊ
6
6
mol����3������ijѹǿ�㶨���ܱ������м���2mol N2��4mol H2���������·�Ӧ��
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol�ﵽƽ��ʱ�����Ϊ��Ӧǰ������֮������
�ٴﵽƽ��ʱ��N2��ת����Ϊ
50%
50%
��������������м���a mol N2��b mol H2��c mol NH3����a��b��c����0������ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ���������ƽ����ͬ���ԱȽϷ�Ӧ�ų�����������
��
��
�ڣ����������������=����������2mol N2��4mol H2������ʼ�����ͬ�ĺ��������У��������ͬ���¶��´ﵽƽ�⣮
���ԱȽ�ƽ��ʱNH3��Ũ�ȣ���
��
��
�����������������=��������������1���ٰ�����ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ���������笠����Ӻ����������ӣ�
����������ˮ���룻
����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ˮ���ʹ����Һ�����ԣ���ϵ���غ�ȷ������Ũ�ȴ�С��
��2����ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�Ϊ����泥�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75��������������ͷ����ļ�����֮����1��0.75=4��3����ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA�������ͷ���淋ļ�����֮��=
��0.3mol=4��3������ԭ���غ�֪�����ɵ���һ��������NF3��
��3�����ں�ѹ���ܱ������У������������ʵ���֮�ȵ��������֮�ȣ����ò���������μӷ�Ӧ�ĵ������ٽ��ת���ʽ��м��㣻
����ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ��������ƽ����ͬ��˵����ƽ��Ϊ��ȫ��Чƽ�⣬����ת���ʱȢ��еĸߣ��μӷ�Ӧ�ĵ��������ʵ����Ϣڶࣻ
�ۺ��������µ�ת���ʽ�С��������Ũ�Ƚ�С��
����������ˮ���룻
����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ˮ���ʹ����Һ�����ԣ���ϵ���غ�ȷ������Ũ�ȴ�С��
��2����ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�Ϊ����泥�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75��������������ͷ����ļ�����֮����1��0.75=4��3����ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA�������ͷ���淋ļ�����֮��=
| 8.96L |
| 22.4L/mol |
��3�����ں�ѹ���ܱ������У������������ʵ���֮�ȵ��������֮�ȣ����ò���������μӷ�Ӧ�ĵ������ٽ��ת���ʽ��м��㣻
����ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ��������ƽ����ͬ��˵����ƽ��Ϊ��ȫ��Чƽ�⣬����ת���ʱȢ��еĸߣ��μӷ�Ӧ�ĵ��������ʵ����Ϣڶࣻ
�ۺ��������µ�ת���ʽ�С��������Ũ�Ƚ�С��
����⣺��1���ٰ�����ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ���������笠����Ӻ����������ӣ���Ӧ����ʽΪ��NH3+H2O?NH3?H2O?NH4++OH-���ʴ�Ϊ��NH3+H2O?NH3?H2O?NH4++OH-��
�ڰ�ˮ��Һ�ʼ��ԣ�����ˮ���룬����c��OH-����10-7mol/L���ʴ�Ϊ������
����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ˮ���ʹ����Һ�����ԣ�����c��H+����c��OH-���������Һ�е���غ��c��Cl-����c��NH4+����������Һ������Ũ�ȴ�С˳���ǣ�c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��2����ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�Ϊ����泥�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75��������������ͷ����ļ�����֮����1��0.75=4��3����ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA�������ͷ���淋ļ�����֮��=
��0.3mol=4��3������ԭ���غ�֪�����ɵ���һ��������NF3��
��ͭ�����������£������ͷ�����Ӧ���ɷ���狀�������������Ӧ����ʽΪ��4NH3+3F2
NF3+3NH4F������Ҫע����ͭ����
�ʴ�Ϊ��4NH3+3F2
NF3+3NH4F������Ҫע����ͭ����
���ڱ�״���£�ÿ����1mol B��ת�Ƶ��ӵ����ʵ���=1mol��3+3��=6mol���ʴ�Ϊ��6��
��3�����ں�ѹ���ܱ������У������������ʵ���֮�ȵ��������֮�ȣ���Ӧ��������ٵ����ʵ���=
(2+4)mol=2mol�����ݷ���ʽ֪���μӷ�Ӧ�ĵ��������ʵ���=���������ٵ����ʵ�����һ��=1mol�����Ե�����ת����=
��100%=50%���ʴ�Ϊ��50%��
����ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ��������ƽ����ͬ��˵����ƽ��Ϊ��ȫ��Чƽ�⣬ƽ��ʱ���������ʵ�����ͬ�����ڢ�����ʼ����cmol NH3���ʢ��з�Ӧ���ɵİ����Ȣ��٣����вμӷ�Ӧ�ĵ��������ʵ����Ϣڶ࣬���Էų��������٣���2�����ʴ�Ϊ������
�ۺ��������·�Ӧ�����ŷ�Ӧ�Ľ��У���������ʵ�����С����ѹǿ��С������ƽ��������Ӧ�����ƶ������Ժ��������µ�ת���ʽ�С��������Ũ�Ƚ�С���ʴ�Ϊ������
�ڰ�ˮ��Һ�ʼ��ԣ�����ˮ���룬����c��OH-����10-7mol/L���ʴ�Ϊ������
����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ˮ���ʹ����Һ�����ԣ�����c��H+����c��OH-���������Һ�е���غ��c��Cl-����c��NH4+����������Һ������Ũ�ȴ�С˳���ǣ�c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
��2����ͭ�Ĵ������£������ͷ�����Ӧ����A��B��AΪ��Σ�Ϊ����泥�B�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75��������������ͷ����ļ�����֮����1��0.75=4��3����ÿ��Ӧ8.96L��������״������ͬʱ����0.3molA�������ͷ���淋ļ�����֮��=
| 8.96L |
| 22.4L/mol |
��ͭ�����������£������ͷ�����Ӧ���ɷ���狀�������������Ӧ����ʽΪ��4NH3+3F2
| ||
�ʴ�Ϊ��4NH3+3F2
| ||
���ڱ�״���£�ÿ����1mol B��ת�Ƶ��ӵ����ʵ���=1mol��3+3��=6mol���ʴ�Ϊ��6��
��3�����ں�ѹ���ܱ������У������������ʵ���֮�ȵ��������֮�ȣ���Ӧ��������ٵ����ʵ���=
| 1 |
| 3 |
| 1mol |
| 2mol |
����ͬ�����´ﵽƽ��ʱ��������и���ֵ����ʵ��������ƽ����ͬ��˵����ƽ��Ϊ��ȫ��Чƽ�⣬ƽ��ʱ���������ʵ�����ͬ�����ڢ�����ʼ����cmol NH3���ʢ��з�Ӧ���ɵİ����Ȣ��٣����вμӷ�Ӧ�ĵ��������ʵ����Ϣڶ࣬���Էų��������٣���2�����ʴ�Ϊ������
�ۺ��������·�Ӧ�����ŷ�Ӧ�Ľ��У���������ʵ�����С����ѹǿ��С������ƽ��������Ӧ�����ƶ������Ժ��������µ�ת���ʽ�С��������Ũ�Ƚ�С���ʴ�Ϊ������
���������⿼����ۺϣ��漰ƽ���ƶ�ԭ��������Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬��Щ����ѧϰ�ѵ㣬ע��������ݺͺ�ѹ����������ת���ʵIJ�ͬ�㣬Ϊ�״��㣮

��ϰ��ϵ�д�
 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
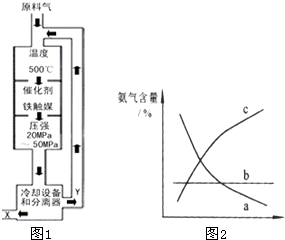 ��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�
��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�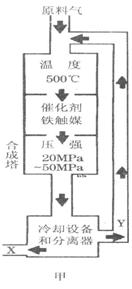
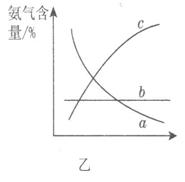
 ��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________�� ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
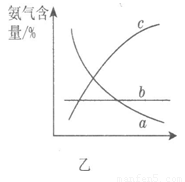
 ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��