��Ŀ����
�����£���0.1000 mol/L HCl��Һ�ζ�20.00 mL 0.1000 mol/L NH3?H2O��Һ���ζ���������ͼ������˵����ȷ����
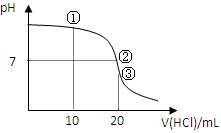

| A������Һ��c(C1��)��c(NH4+)��c(OH��)��c(H+) |
| B������Һ��c(NH4+)=c(C1��)��c(OH��)=c(H+) |
| C������Һ��c(H+)��c(NH3��H2O) + c(OH��) |
| D���ζ������п��ܳ��֣�c(NH3?H2O)��c(NH4+)��c(OH��)��c(Cl��)��c(H+) |
BD
���ݵ���غ�c(C1��)��c(OH��)��c(NH4+)��c(H+)��֪��A����ȷ���ڱ�ʾ��Һ�����ԣ����ݵ���غ���жϣ�B��ȷ���۱�ʾ����Ͱ�ˮǡ�÷�Ӧ����Һ��ֻ���Ȼ�泥�ˮ�������ԡ����������غ��֪��c(H+)��c(NH3��H2O) + c(OH��)��C����ȷ����������������ʱ�����ܳ���ѡ��D�еĹ�ϵ�����Դ�ѡBD��

��ϰ��ϵ�д�
�����Ŀ
 ������5�����е�
������5�����е� ��ӽ���
��ӽ���