��Ŀ����
��2011?��������ģ��H2O2����Ҫ�Ļ�ѧ�Լ�����ʵ���Һ�����ʵ����Ӧ�ù㷺��
��Ӧ���䲻�ȶ��ԣ�H2O2�ɱ������о������Է�Ӧ���ʵ�Ӱ�죮
��1��д��H2O2��MnO2���·ֽ�Ļ�ѧ����ʽ
��2��H2O2��������Ҫ�ϸ�������Fe2+��Fe3+������ᷢ������������Ӧ���ӿ���ֽ⣬��Fe2+��Fe3+���Ե������ֲ��䣮
��2Fe2++H2O2+2H+�T2Fe3++2H2O
��
��3���ӣ�1���ͣ�2������������������Ԫ����Ѱ��H2O2�ֽ�Ĵ��������о���ֵ
a������Ԫ��b������Ԫ��c������Ԫ��
��H2O2��ˮ��Һ�������ԣ�д��һ����������ȡH2O2�Ļ�ѧ����ʽ
��H2O2��ʹ����ͨ�����������Ⱦ��
��1����ҵ������Cl2+H2O2�T2HCl+O2���ȣ��ڴ˷�Ӧ�б�������������
��2������ij��Һ���軯���CN-����������з�Ӧ��CN-+H2O2
����ʵ���ҳ�������KMnO4��Һ�ⶨH2O2��Ũ�ȣ�
ȡ10.00mLH2O2��Һ��Ʒ���ܶȽ���Ϊ1g/mL������ƿ�У���ˮ��50.00mL��������0.10mol/L KMnO4��Һ40.00mL����ζ��յ㣮��ԭ��Ʒ��H2O2������������
����֪��2KMnO4+5H2O2+3H2SO4�TK2SO4+2MnSO4+5O2��+8H2O����
��Ӧ���䲻�ȶ��ԣ�H2O2�ɱ������о������Է�Ӧ���ʵ�Ӱ�죮
��1��д��H2O2��MnO2���·ֽ�Ļ�ѧ����ʽ
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��2��H2O2��������Ҫ�ϸ�������Fe2+��Fe3+������ᷢ������������Ӧ���ӿ���ֽ⣬��Fe2+��Fe3+���Ե������ֲ��䣮
��2Fe2++H2O2+2H+�T2Fe3++2H2O
��
2Fe3++H2O2=2Fe2++O2��+2H+
2Fe3++H2O2=2Fe2++O2��+2H+
�������ӷ���ʽ����3���ӣ�1���ͣ�2������������������Ԫ����Ѱ��H2O2�ֽ�Ĵ��������о���ֵ
b
b
��a������Ԫ��b������Ԫ��c������Ԫ��
��H2O2��ˮ��Һ�������ԣ�д��һ����������ȡH2O2�Ļ�ѧ����ʽ
Na2O2+2HCl=2NaCl+H2O2
Na2O2+2HCl=2NaCl+H2O2
����H2O2��ʹ����ͨ�����������Ⱦ��
��1����ҵ������Cl2+H2O2�T2HCl+O2���ȣ��ڴ˷�Ӧ�б�������������
H2O2
H2O2
����2������ij��Һ���軯���CN-����������з�Ӧ��CN-+H2O2
=HCO3-
=HCO3-
+NH3������ʵ���ҳ�������KMnO4��Һ�ⶨH2O2��Ũ�ȣ�
ȡ10.00mLH2O2��Һ��Ʒ���ܶȽ���Ϊ1g/mL������ƿ�У���ˮ��50.00mL��������0.10mol/L KMnO4��Һ40.00mL����ζ��յ㣮��ԭ��Ʒ��H2O2������������
3.4%��
3.4%��
������֪��2KMnO4+5H2O2+3H2SO4�TK2SO4+2MnSO4+5O2��+8H2O����
��������1�����������ڶ�������������������������ˮ��������
��2�����ݹ�������������������л�ԭ�ԣ�
��3������MnԪ�غ�FeԪ�ض����ڹ���Ԫ�أ�
������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2��
��1�����ݻ��ϼ۵ı仯�жϷ�Ӧ���ͣ�
��2������ԭ���غ�͵���غ������
�������ݷ�Ӧ��ԭ������ʽ���м��㣮
��2�����ݹ�������������������л�ԭ�ԣ�
��3������MnԪ�غ�FeԪ�ض����ڹ���Ԫ�أ�
������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2��
��1�����ݻ��ϼ۵ı仯�жϷ�Ӧ���ͣ�
��2������ԭ���غ�͵���غ������
�������ݷ�Ӧ��ԭ������ʽ���м��㣮
����⣺��1�����������ڶ�������������������������ˮ��������˫��ˮ�ֽ�Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2�����ʴ�Ϊ��2H2O2
2H2O+O2����
��2�����������������ԣ�������Fe2+��2Fe2++H2O2+2H+�T2Fe3++2H2O�����л�ԭ�ԣ��ܱ�Fe3+������2Fe3++H2O2=2Fe2++O2��+2H+���ʴ�Ϊ��2Fe3++H2O2=2Fe2++O2��+2H+����
��3��MnԪ�غ�FeԪ�ض����ڹ���Ԫ�أ����Թ���Ԫ����ΪH2O2�ֽ�Ĵ��������о���ֵ��
��ѡ��b��
������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2������ʽΪ��Na2O2 +2HCl=2NaCl+H2O2��
�ʴ�Ϊ��Na2O2 +2HCl=2NaCl+H2O2��
��1��Cl2+H2O2�T2HCl+O2���÷�Ӧ��Ԫ�صĻ��ϼ۱仯Ϊ��H2O2��O2��OԪ����-1�ۡ�0�ۣ�ʧ���ӣ�����H2O2�ǻ�ԭ������ԭ������������������Ӧ��
�ʴ�Ϊ��H2O2��
��2����ԭ���غ�͵���غ�ã�CN-+H2O2=HCO3-+NH3�����ʴ�Ϊ��HCO3-��
�������ĸ������������0.1mol/L��0.04L=0.004mol����˫��ˮ�����ʵ���Ϊn����
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.004mol n
���n=0.01mol������˫��ˮ������Ϊ��0.01mol��34g/mol=0.34g��
10.00mLH2O2��Һ��Ʒ������Ϊ10g��˫��ˮ����������=
��100%=3.4%��
�ʴ�Ϊ��3.4%��
| ||
| ||
��2�����������������ԣ�������Fe2+��2Fe2++H2O2+2H+�T2Fe3++2H2O�����л�ԭ�ԣ��ܱ�Fe3+������2Fe3++H2O2=2Fe2++O2��+2H+���ʴ�Ϊ��2Fe3++H2O2=2Fe2++O2��+2H+����
��3��MnԪ�غ�FeԪ�ض����ڹ���Ԫ�أ����Թ���Ԫ����ΪH2O2�ֽ�Ĵ��������о���ֵ��
��ѡ��b��
������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2������ʽΪ��Na2O2 +2HCl=2NaCl+H2O2��
�ʴ�Ϊ��Na2O2 +2HCl=2NaCl+H2O2��
��1��Cl2+H2O2�T2HCl+O2���÷�Ӧ��Ԫ�صĻ��ϼ۱仯Ϊ��H2O2��O2��OԪ����-1�ۡ�0�ۣ�ʧ���ӣ�����H2O2�ǻ�ԭ������ԭ������������������Ӧ��
�ʴ�Ϊ��H2O2��
��2����ԭ���غ�͵���غ�ã�CN-+H2O2=HCO3-+NH3�����ʴ�Ϊ��HCO3-��
�������ĸ������������0.1mol/L��0.04L=0.004mol����˫��ˮ�����ʵ���Ϊn����
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.004mol n
���n=0.01mol������˫��ˮ������Ϊ��0.01mol��34g/mol=0.34g��
10.00mLH2O2��Һ��Ʒ������Ϊ10g��˫��ˮ����������=
| 0.34g |
| 10g |
�ʴ�Ϊ��3.4%��
������������H2O2�����ʣ�������ѧ���������⡢���������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ






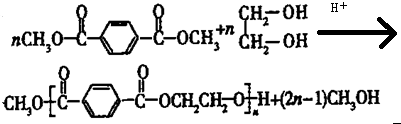
 �ϳ�
�ϳ� ������ͼ����ע����Ӧ������
������ͼ����ע����Ӧ������