��Ŀ����
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ������1��W��Ԫ�����ڱ��е�λ����
�ڶ�����IVA��
�ڶ�����IVA��
��Z2Y�ĵ���ʽ��

��2������֪�������������������ķ�Ӧһ����������ķ�Ӧ����������ѧ֪ʶ�ж����·�Ӧ2WY��g��=2W��s��+Y2��g����H��0���ڳ�����
����
����
�Է����У���ܡ����ܡ�������400��ʱ����0.5L�ķ�Ӧ��������X2��Q2��Ӧ�ϳ�XQ3��Ӧ���ﵽƽ��ʱ�����X2��Q2��XQ3�����ʵ����ֱ�Ϊ2mol��1mol��2mol����˷�Ӧ��400��ʱ�Ļ�ѧƽ�ⳣ��Ϊ
0.5
0.5
��3��2.24L����״����XQ3��200mL 1mol/L QXY3��Һ���պ�������Һ������Ũ�ȴӴ�С��˳����
c��NO3-����c��H+����c��NH4+����c��OH-��
c��NO3-����c��H+����c��NH4+����c��OH-��
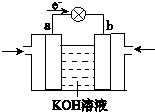
����4��WQ4Y��Y2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��a���ĵ缫��Ӧʽ��
CH3OH+8OH--6e-=CO32-+6H2O
CH3OH+8OH--6e-=CO32-+6H2O
��5��X��Z��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ��
Na3N+4H2O=3NaOH+NH3?H2O
Na3N+4H2O=3NaOH+NH3?H2O
��������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�ӦΪH��Q��W��ɵĻ�������һ���������壬������ΪCH4����WΪC��
W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�ΪCO��NOx����YΪO��XΪN��Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ�������������ӻ�����ֱ�ΪNa2O��Na2O2����ΪZΪNa��
��1��WΪC��λ�����ڱ��ڶ�����IVA�壬Z2YΪNa2O��Ϊ���ӻ����
��2�������������оݡ�G=��H-T?��S�жϷ�Ӧ�ڳ������ܷ���У�����k=
���㣻
��3��n��NH3��=
=0.1mol��n��HNO3��=0.2L��1mol/L=0.2mol������������������ж�����Ũ�ȴ�С��
��4��CH3OH����ȼ�ϵ���У�CH3OHΪ��صĸ�����Ӧ��
��5��X��Z��ɵ�һ�����ӻ�����ΪNa3N������ˮ��Ӧ�������ּ������ΪNaOH��NH3?H2O��
W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�ΪCO��NOx����YΪO��XΪN��Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ�������������ӻ�����ֱ�ΪNa2O��Na2O2����ΪZΪNa��
��1��WΪC��λ�����ڱ��ڶ�����IVA�壬Z2YΪNa2O��Ϊ���ӻ����
��2�������������оݡ�G=��H-T?��S�жϷ�Ӧ�ڳ������ܷ���У�����k=
| c2(NH3) |
| c(N2)��c3(H2 ) |
��3��n��NH3��=
| 2.24L |
| 22.4L/mol |
��4��CH3OH����ȼ�ϵ���У�CH3OHΪ��صĸ�����Ӧ��
��5��X��Z��ɵ�һ�����ӻ�����ΪNa3N������ˮ��Ӧ�������ּ������ΪNaOH��NH3?H2O��
����⣺Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�ӦΪH��Q��W��ɵĻ�������һ���������壬������ΪCH4����WΪC��W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�ΪCO��NOx����YΪO��XΪN��Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ�������������ӻ�����ֱ�ΪNa2O��Na2O2����ΪZΪNa����
��1��WΪC��ԭ������Ϊ6��ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6��Ӧλ�����ڱ��ڶ�����IVA�壬
Z2YΪNa2O��Ϊ���ӻ��������ʽΪ ��
��
�ʴ�Ϊ���ڶ�����IVA�壻 ��
��
��2�������������оݡ�G=��H-T?��S����֪����H��T?��Sʱ��Ӧ�����Է����У��������ڽϸߵ��¶��²ſ��Խ��У���ѧ��Ӧ�ܷ��Է�����ȡ�����ʱ���ر���ۺ��оݣ�����ֻ���ʱ���ر����������ʴ�Ϊ�����ܣ�
�ڴ�ƽ��ʱ�������ʵ�Ũ�ȷֱ�Ϊ��c��N2��=4mol/L��c��H2��=2mol/L��c��NH3��=4mol/L��
��k=
=
=0.5���ʴ�Ϊ��0.5��
��3��n��NH3��=
=0.1mol��n��HNO3��=0.2L��1mol/L=0.2mol��HNO3����������NH4NO3���������ʵ�����ȣ�������NH4+ˮ�⣬��c��H+����c��NH4+����������Һ�д���c��NO3-����c��H+����c��NH4+����c��OH-����
�ʴ�Ϊ��c��NO3-����c��H+����c��NH4+����c��OH-����
��4��CH3OH����ȼ�ϵ���У�CH3OHΪ��صĸ�����Ӧ�����������缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O��
��5��X��Z��ɵ�һ�����ӻ�����ΪNa3N������ˮ��Ӧ�������ּ������ΪNaOH��NH3?H2O����Ӧ�Ļ�ѧ����ʽΪNa3N+4H2O=3NaOH+NH3?H2O��
�ʴ�Ϊ��Na3N+4H2O=3NaOH+NH3?H2O��
��1��WΪC��ԭ������Ϊ6��ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6��Ӧλ�����ڱ��ڶ�����IVA�壬
Z2YΪNa2O��Ϊ���ӻ��������ʽΪ
 ��
���ʴ�Ϊ���ڶ�����IVA�壻
 ��
�� ��2�������������оݡ�G=��H-T?��S����֪����H��T?��Sʱ��Ӧ�����Է����У��������ڽϸߵ��¶��²ſ��Խ��У���ѧ��Ӧ�ܷ��Է�����ȡ�����ʱ���ر���ۺ��оݣ�����ֻ���ʱ���ر����������ʴ�Ϊ�����ܣ�
�ڴ�ƽ��ʱ�������ʵ�Ũ�ȷֱ�Ϊ��c��N2��=4mol/L��c��H2��=2mol/L��c��NH3��=4mol/L��
��k=
| c2(NH3) |
| c(N2)��c3(H2 ) |
| 42 |
| 4��23 |
��3��n��NH3��=
| 2.24L |
| 22.4L/mol |
�ʴ�Ϊ��c��NO3-����c��H+����c��NH4+����c��OH-����
��4��CH3OH����ȼ�ϵ���У�CH3OHΪ��صĸ�����Ӧ�����������缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O��
��5��X��Z��ɵ�һ�����ӻ�����ΪNa3N������ˮ��Ӧ�������ּ������ΪNaOH��NH3?H2O����Ӧ�Ļ�ѧ����ʽΪNa3N+4H2O=3NaOH+NH3?H2O��
�ʴ�Ϊ��Na3N+4H2O=3NaOH+NH3?H2O��
���������⿼��Ԫ�ص��ƶϣ���Ŀ�Ѷ��еȣ���������ڵ绯ѧ����Һ�е�ˮ���������ʵĵ����֪ʶ�Ŀ��飬��Ŀ�ۺ϶Ƚϸߣ�����ʱע��������֪ʶ�ͷ�����Ӧ�ã�Ϊ�߿������鷽ʽ��

��ϰ��ϵ�д�
�����Ŀ
Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X����ɫ��Ӧ�ʻ�ɫ��QԪ�ص�ԭ�����������������ڲ��������2����W��Z������������ͬ��Z�ĺ˵������W��2����Ԫ��Y�ĺϽ����ճ�������ʹ�ù㷺�Ľ�������֮һ������˵����ȷ���ǣ�������
| A���⻯���ȶ��ԣ�Z��W | B��ԭ�Ӱ뾶�Ĵ�С˳��rX��rY��rQ��rW | C��Ԫ��Q��Z���γ�QZ2�͵Ĺ��ۻ����� | D��X��Y������������ˮ����֮�䲻�ܷ�����Ӧ |
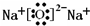
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮