��Ŀ����
�״��ϳɷ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
CH3OH(g) ��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
(1)�Ʊ��ϳ�����CH4+H2O��g��![]() CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO2+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м��������
CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO2+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м�������� ��̼�����Ϊ__________��
��̼�����Ϊ__________��
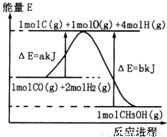
(2)�ϳɼ״����ٷ�Ӧ���������������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________ ��
ʵ������1L�ܱ������н���ģ��ϳ�ʵ�顣��lmolCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£�
 (�������ݵ�λ��mol��L��1)
(�������ݵ�λ��mol��L��1)
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��500��ʱƽ�ⳣ��K����ֵΪ___________��
��300��ʱ�����������ݻ�ѹ����ԭ����1��2���������������������£���ƽ����ϵ ������Ӱ����__________(����ĸ)��
a.c(H2)��С b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c.CH3OH�����ʵ������� d������ƽ��ʱc(H2)��c(CH3OH)��С
 ��3���״�ֱ��ȼ�ջ����һ������Ⱦ��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�״�ȼ�ϵ�أ� �õ�ظ����ĵ缫��ӦʽΪ ������һ��ʱ���9��6g�״���ȫ��Ӧʱ���� NA������ת�ơ�
��3���״�ֱ��ȼ�ջ����һ������Ⱦ��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�״�ȼ�ϵ�أ� �õ�ظ����ĵ缫��ӦʽΪ ������һ��ʱ���9��6g�״���ȫ��Ӧʱ���� NA������ת�ơ�
��1��3:1��2�֣�
��2����![]()
![]() ��2�֣�
��2�֣�
��0.080mol/(L��min)(2��) ��25��2�֣� ��c��d��2�֣�
��3��CH3OH+8OH--6e-=CO32-+6H2O (2��) 1.8 ��2�֣�

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д���1���Ʊ��ϳ�����CH4+H2O��g��?CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO2+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м����������̼�����Ϊ______��
��2���ϳɼ״����ٷ�Ӧ���������������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ______��
ʵ������1L�ܱ������н���ģ��ϳ�ʵ�飮��1molCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£�
| 10min | 20min | 30min | 40min | 50min | 60min | |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
��500��ʱƽ�ⳣ��K����ֵΪ______��
��300��ʱ�����������ݻ�ѹ����ԭ����
 ���������������������£���ƽ����ϵ������Ӱ����______������ĸ����
���������������������£���ƽ����ϵ������Ӱ����______������ĸ����a��c��H2����С b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c��CH3OH�����ʵ�������d������ƽ��ʱc��H2��/c��CH3OH����С��
 ��2009?���ڶ�ģ���״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ���Σ�
��2009?���ڶ�ģ���״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ���Σ� �״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
�״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���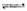 CH3OH(g)
CH3OH(g)
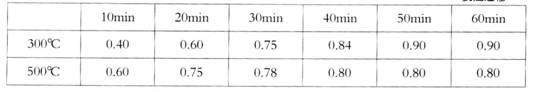 ��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��