��Ŀ����
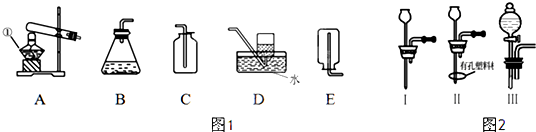
ij����С���ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã��г������ԣ����������������ʵ��
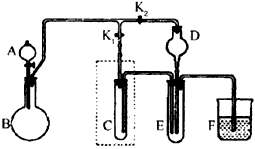
(1)ʵ��1����ȡ���ռ�H2��Cl2��
������ȡ���ռ�H2��������K1Ӧ��___________������رա���������K2Ӧ��_________������رա�����H2�ռ�������_______�����ʾ������Ӣ����ĸ���С�
�������Թ�C�м���ij��Һ�壬�������ռ�Cl2������Һ�����ѡ��____�����ţ���
a.����NaHCO3��Һ b.����NaCl��Һ c.NaOH��Һ
(2)ʵ��2���øɡ�ʪ������ɫ������֤����Cl2������Ư���Զ���ʪ��Cl2����Ư���ԡ�
�����ø��������Ũ���ᷴӦ��ȡCl2��ʵ��ǰ�Ƚ�װ��C��Ϊ��ͼ��ʾװ�ã���Ӧ��D�м���_____������ţ���
a.Ũ���� b.�Ȼ��� c.��ʯ�� d.̼������
������ȡ���ռ�H2��������K1Ӧ��___________������رա���������K2Ӧ��_________������رա�����H2�ռ�������_______�����ʾ������Ӣ����ĸ���С�
�������Թ�C�м���ij��Һ�壬�������ռ�Cl2������Һ�����ѡ��____�����ţ���
a.����NaHCO3��Һ b.����NaCl��Һ c.NaOH��Һ
(2)ʵ��2���øɡ�ʪ������ɫ������֤����Cl2������Ư���Զ���ʪ��Cl2����Ư���ԡ�
�����ø��������Ũ���ᷴӦ��ȡCl2��ʵ��ǰ�Ƚ�װ��C��Ϊ��ͼ��ʾװ�ã���Ӧ��D�м���_____������ţ���
a.Ũ���� b.�Ȼ��� c.��ʯ�� d.̼������

����ʪ����ɫ����Ӧ����_______�����ʾ������Ӣ����ĸ���У��������ɫ����Ӧ����______�����ʾ������Ӣ����ĸ���С�
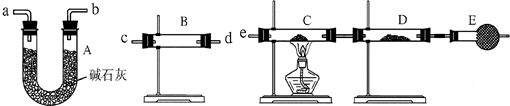
(1)�ٴ��رգ�C����b
(2)��b����C��E
(2)��b����C��E

��ϰ��ϵ�д�
�����Ŀ
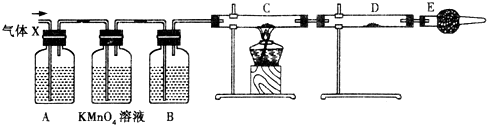
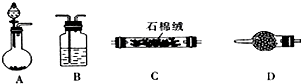
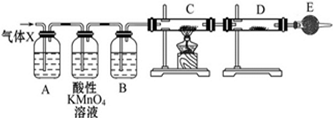 ij����С���ͬѧ����ʵ������п��Ũ���ᷴӦ��ʵ��ʱ����ͬѧ��Ϊ�����������SO2�⣬�����ܲ�����������ͬѧΪ�˼��������ж��Ƿ���ȷ���������ͼ��ʾ��ʵ��װ�ã����У�װ��B��ʢ��Ũ���ᣬװ��C�з��ú�ɫCuO��ĩ��װ��D�з��õ�����ˮ����ͭ��ĩ��п��Ũ���Ṳ��ʱ����������ΪX���Ҹ�װ����ȥ�����Իش�
ij����С���ͬѧ����ʵ������п��Ũ���ᷴӦ��ʵ��ʱ����ͬѧ��Ϊ�����������SO2�⣬�����ܲ�����������ͬѧΪ�˼��������ж��Ƿ���ȷ���������ͼ��ʾ��ʵ��װ�ã����У�װ��B��ʢ��Ũ���ᣬװ��C�з��ú�ɫCuO��ĩ��װ��D�з��õ�����ˮ����ͭ��ĩ��п��Ũ���Ṳ��ʱ����������ΪX���Ҹ�װ����ȥ�����Իش�