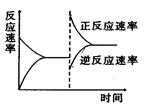
��Ŀ����
��1���̶�������CO2����Ч��������Դ�������ٿ����е��������塣��ҵ����һ����CO2�������״�ȼ�ϵķ�����CO2(g)��3H2(g) CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��
CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��
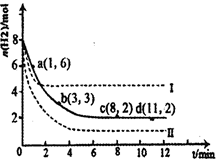
������ʱ���ƽ����Ӧ����������__________����С����______________��
�ڽ��ı�ijһʵ�������ٽ�������ʵ����H2�����ʵ�����ʱ��仯��ͼ��������ʾ�����ߢ��Ӧ��ʵ��ı��������________�����ߢ��Ӧ��ʵ��ı��������_________��
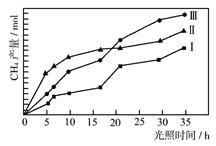
��2�����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ��������CO2��H2O(g)�ڲ�ͬ����(��)�����£�CH4���������ʱ��ı仯��ͼ��ʾ����0~30 h�ڣ�CH4��ƽ����������v(��)��v(��)��v(��)�Ӵ�С��˳��Ϊ ����Ӧ��ʼ���12Сʱ�ڣ��ڵ�___________�ִ����������£��ռ���CH4��ࡣ
 CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��
CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��
������ʱ���ƽ����Ӧ����������__________����С����______________��
| A��0��1min | B��1��3min | C��3��8min | D��8��11min |
��2�����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ��������CO2��H2O(g)�ڲ�ͬ����(��)�����£�CH4���������ʱ��ı仯��ͼ��ʾ����0~30 h�ڣ�CH4��ƽ����������v(��)��v(��)��v(��)�Ӵ�С��˳��Ϊ ����Ӧ��ʼ���12Сʱ�ڣ��ڵ�___________�ִ����������£��ռ���CH4��ࡣ

��12�֣���1����A��D �������¶ȣ�����ѹǿ����������CO2Ũ�ȣ���2�֣�
��2��v(��)>v(��)>v(��)��2�֣�;�� ��2�֣�
��2��v(��)>v(��)>v(��)��2�֣�;�� ��2�֣�
�����������1������ͼ1��֪��0��1min�������ı仯��Ϊ8mol-6mol=2mol��
B��1��3min�������ı仯��Ϊ6mol-3mol=3mol��ƽ��1min�仯��Ϊ1.5mol��
C��3��8min�������ı仯��Ϊ3mol-2mol=1mol��ƽ��1min�仯��Ϊ0.2mol��
D��8��11min��ƽ��״̬�����������ʵ������ٱ仯��
��1��3min��������8��11min��������
�ʴ�Ϊ��A��D��
�ڶ��ڿ��淴ӦCO2��g��+3H2��g��
 CH3OH��g��+H2O��g����H=-49.0kJ��mol��1������Ӧ�������С�ķ��ȷ�Ӧ��
CH3OH��g��+H2O��g����H=-49.0kJ��mol��1������Ӧ�������С�ķ��ȷ�Ӧ����ͼ1��֪�����ߢ����ȵ���ƽ�⣬ƽ��ʱ���������ʵ������ʸı�����Ӧ����Ӧ������ƽ�����淴Ӧ�ƶ������Բ�ȡ�Ĵ�ʩΪ�������¶ȣ�
���ߢ�ƽ���ʱ���ԭƽ��̣�ƽ��ʱ���������ʵ�����С���ʸı�����Ӧ����Ӧ������ƽ��������Ӧ�ƶ������Բ�ȡ�Ĵ�ʩΪ������ѹǿ����������CO2Ũ�ȣ�
�ʴ�Ϊ�������¶ȣ�����ѹǿ����������CO2Ũ�ȣ�
��2����ͼ2��֪����0��30h�ڣ���������ʵ����仯��Ϊ��n������n������n��������0��30h�ڣ�CH4��ƽ����������v����v����v����
��ͼ2��֪��Ӧ��ʼ���12Сʱ�ڣ��ڵڢ��ִ����������£��ռ���CH4��࣮
�ʴ�Ϊ��v����v����v������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2NH3(g) ��H=һ92. 4 KJ��mol-1
2NH3(g) ��H=һ92. 4 KJ��mol-1
 2SO3(g)����H��0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����
2SO3(g)����H��0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����

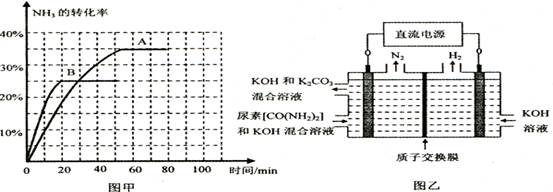
 CH3OCH3(g)+H2O(g)��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)+H2O(g)��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£� 3F+2G���ô�������ø÷�Ӧ�ڲ�ͬpH�����£�����D��Ũ�ȣ���ÿ����Һ�������ʵ�������ʾ����λΪmg/L���仯��ͼ��ʾ�������й�˵����ȷ����
3F+2G���ô�������ø÷�Ӧ�ڲ�ͬpH�����£�����D��Ũ�ȣ���ÿ����Һ�������ʵ�������ʾ����λΪmg/L���仯��ͼ��ʾ�������й�˵����ȷ����
 2SO3(g) ��H<0�������й�������ȷ����
2SO3(g) ��H<0�������й�������ȷ���� 2NH3(g) ��H=��92kJ/mol����ֻ�ı�����һ��������һ������������Ӧ������ʹƽ��������Ӧ�����ƶ����ǣ� ��
2NH3(g) ��H=��92kJ/mol����ֻ�ı�����һ��������һ������������Ӧ������ʹƽ��������Ӧ�����ƶ����ǣ� �� Si (s)+ 4HCl(g) ��236kJ
Si (s)+ 4HCl(g) ��236kJ