��Ŀ����
NiSO4?6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����ɵ�Ʒ������������⣬�����У�Cu��Zn��Fe��Cr��Ԫ�صĻ��������ʣ�Ϊԭ�ϻ�á��йع����������£�
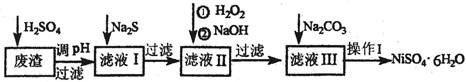
��1���������м�H2SO4������Ҫ��ֽ��裬��Ŀ���� ��
��2����Na2S��Ŀ���dz�ȥͭ��п�����ʣ���д����ȥCu2+�����ӷ���ʽ ��
��3����6%��H2O2ʱ���¶Ȳ��ܹ��ߣ���Ŀ���� ��
��4��������������H2O2�������������NaOH����pHֵ2��4��Χ�������������������������������У�����������NaClO3���棬��NaClO3����Fe2+�����ӷ���ʽΪ ��
��5��������������ҺIII�����ʵ���Ҫ�ɷ��ǣ� ��
��6������I�������¹��̣����ˣ���H2SO4�ܽ⣬ �� �����ˡ�ϴ�ӻ�ò�Ʒ��

��1���������м�H2SO4������Ҫ��ֽ��裬��Ŀ���� ��
��2����Na2S��Ŀ���dz�ȥͭ��п�����ʣ���д����ȥCu2+�����ӷ���ʽ ��
��3����6%��H2O2ʱ���¶Ȳ��ܹ��ߣ���Ŀ���� ��
��4��������������H2O2�������������NaOH����pHֵ2��4��Χ�������������������������������У�����������NaClO3���棬��NaClO3����Fe2+�����ӷ���ʽΪ ��
��5��������������ҺIII�����ʵ���Ҫ�ɷ��ǣ� ��
��6������I�������¹��̣����ˣ���H2SO4�ܽ⣬ �� �����ˡ�ϴ�ӻ�ò�Ʒ��
��16�֣���1���ӿ췴Ӧ���ʻ���߽����ʣ������ĸ��֣� ��2�֣�
��2��S2��+Cu2+=CuS����2�֣�
��3�����٣���ֹ����������ķֽ� ��2�֣�
��4��6Fe2++ClO3��+6H+=6Fe3++Cl��+3H2O��2�֣�
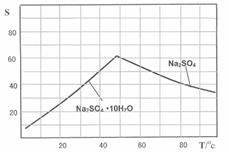
��5��Na2SO4��NiSO4��4�֣�©ѡ��2�֣���ѡ�����֣�
��6������Ũ�� ��ȴ�ᾧ��4�֣�
��2��S2��+Cu2+=CuS����2�֣�
��3�����٣���ֹ����������ķֽ� ��2�֣�
��4��6Fe2++ClO3��+6H+=6Fe3++Cl��+3H2O��2�֣�
��5��Na2SO4��NiSO4��4�֣�©ѡ��2�֣���ѡ�����֣�
��6������Ũ�� ��ȴ�ᾧ��4�֣�
�����������1�����ݻ�ѧ��Ӧ���ʵ�Ӱ�����ؼ����ɿ�֪����ֽ����Ŀ���Ǽӿ�����е������ʵ������ᷴӦ�����ʣ������Ԫ�صĽ����ʣ���2��Na2S�ǿ������Σ�����ˮʱ�����S2����S2����Cu2+��Ӧ����CuS��������3��������������ɫ����������Ŀ���ǽ�Fe2+��ȫ����ΪFe3+���Ҳ������µ����ʣ�����H2O2���ȶ��������ֽ�Ϊˮ������������¶Ȳ��ܹ��ߣ���4��NaClO3�ǿ������Σ�NaOH��ǿ����������֪��Fe2+�ǻ�ԭ����ClO3������������Fe3+�����������ԭ������Cl����������Cl2����Ϊ������Һ���ܴ�������Cl2��������Ԫ�ػ��ϼ���������������Ԫ�ػ��ϼ۽�������������غ㡢ԭ���غ�ԭ���ɵ÷�Ӧʽ��6Fe2++ClO3��+6H+=6Fe3++Cl��+3H2O����5����ͼ��֪����Na2S��Ŀ���dz�ȥͭ��п�����ʣ���H2O2��NaOH��Ŀ���dz�ȥ�������ʣ���Na2CO3��Ŀ���dz�ȥ�������ʣ�����ƶ���Һ1����Ҫ�ɷ���NiSO4��FeSO4��Cr2(SO4)3��Na2SO4����ҺII����Ҫ�ɷ���NiSO4��Cr2(SO4)3��Na2SO4����ҺIII����Ҫ�ɷ���NiSO4��Na2SO4����6����NiSO4��Na2SO4������Ļ����Һ����ȡNiSO4?6H2O���壬��Ҫ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȹ��̡�

��ϰ��ϵ�д�
�����Ŀ
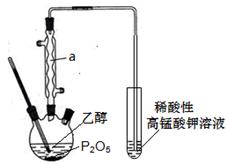

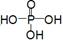 ��__________________________________��
��__________________________________��