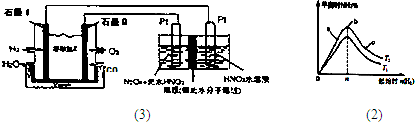
��Ŀ����
��2010?ʯ��ɽ��һģ��A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȣ�
��1��EԪ�������ڱ��е�λ��Ϊ
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ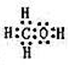
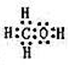 ��
��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⣮�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���Ȼ���DA���۵�Ϊ800��DA����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ�����ȫ�������
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ
��1��EԪ�������ڱ��е�λ��Ϊ
�������ڣ�IIIA��
�������ڣ�IIIA��
����2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ
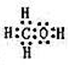
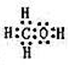
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ
H2O2 H++HO2-
H++HO2-
 H++HO2-
H++HO2-H2O2 H++HO2-
H++HO2-
���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ H++HO2-
H++HO2-BaO2+H2SO4=BaSO4��+H2O2
BaO2+H2SO4=BaSO4��+H2O2
����4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⣮�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ
Cu+2H++H2O2=Cu2++2H2O
Cu+2H++H2O2=Cu2++2H2O
����5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���Ȼ���DA���۵�Ϊ800��DA����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ�����ȫ�������
56L
56L
����״���£�����6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ
3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+
3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+
��������A��W���γ�����Һ̬������A2W��A2W2����Һ̬������ֱ�ΪH2O��H2O2����AΪHԪ�أ�WΪOԪ�أ�A��Dͬ���壬��DΪNaԪ�أ�EԪ�ص���������������������ȣ���E��ԭ���������ӦΪ�������ڣ�IIIA��Ԫ�أ�ΪAlԪ�أ�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39����B��ԭ������Ϊx������1+x+8+11+13=39��x=6������BΪCԪ�أ�
���ԭ�ӽṹ�Լ�Ԫ�ض�Ӧ�ĵ��ʡ�����������ʿɽ����⣮
���ԭ�ӽṹ�Լ�Ԫ�ض�Ӧ�ĵ��ʡ�����������ʿɽ����⣮
����⣺A��W���γ�����Һ̬������A2W��A2W2����Һ̬������ֱ�ΪH2O��H2O2����AΪHԪ�أ�WΪOԪ�أ�A��Dͬ���壬��DΪNaԪ�أ�EԪ�ص���������������������ȣ���E��ԭ���������ӦΪ�������ڣ�IIIA��Ԫ�أ�ΪAlԪ�أ�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39����B��ԭ������Ϊx������1+x+8+11+13=39��x=6������BΪCԪ�أ���
��1��EΪAlԪ�أ�ԭ������Ϊ13��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ3��λ�����ڱ��������ڣ�IIIA�壬
�ʴ�Ϊ���������ڣ�IIIA�壻
��2����A��B��W����Ԫ����ɵ�18���ӵ�����ΪCH3OH��Ϊ���ۻ��������ʽΪ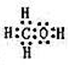 ��
��
�ʴ�Ϊ��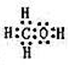 ��
��
��3��Ϊ��Ԫ���ᣬ�Ե�һ������Ϊ��������뷽��ʽΪH2O2 H++HO2-��
H++HO2-��
���ᴦ��BaO2���Ʊ�H2O2������BaSO4���ɣ���Ӧ�Ļ�ѧ����ʽΪBaO2+H2SO4=BaSO4��+H2O2��
�ʴ�Ϊ��H2O2 H++HO2-��BaO2+H2SO4=BaSO4��+H2O2��
H++HO2-��BaO2+H2SO4=BaSO4��+H2O2��
��4��Cu��ϡ�����Ӧ��������������Ե�H2O2������������ͭ��ˮ����Ӧ�����ӷ���ʽΪCu+2H++H2O2=Cu2++2H2O��
�ʴ�Ϊ��Cu+2H++H2O2=Cu2++2H2O��
��5��NaH+H2O=NaOH+H2��
1mol 1mol 1mol
2NaOH+2Al+2H2O=2NaAlO2+3H2����
1mol 1mol 1.5mol
n��H2��=1mol+1.5mol=2.5mol��
V��H2��=2.5mol��22.4L/mol=56L��
�ʴ�Ϊ��56L��
��6��D��ij������ʵ���ɫ��ΪNa2O2�����Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ����������غ��֪������ΪFe��OH��3��NaCl��FeCl3��
��Ӧ�����ӷ���ʽΪ3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+��
�ʴ�Ϊ��3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+��
��1��EΪAlԪ�أ�ԭ������Ϊ13��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ3��λ�����ڱ��������ڣ�IIIA�壬
�ʴ�Ϊ���������ڣ�IIIA�壻
��2����A��B��W����Ԫ����ɵ�18���ӵ�����ΪCH3OH��Ϊ���ۻ��������ʽΪ
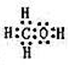 ��
���ʴ�Ϊ��
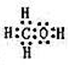 ��
����3��Ϊ��Ԫ���ᣬ�Ե�һ������Ϊ��������뷽��ʽΪH2O2
 H++HO2-��
H++HO2-�����ᴦ��BaO2���Ʊ�H2O2������BaSO4���ɣ���Ӧ�Ļ�ѧ����ʽΪBaO2+H2SO4=BaSO4��+H2O2��
�ʴ�Ϊ��H2O2
 H++HO2-��BaO2+H2SO4=BaSO4��+H2O2��
H++HO2-��BaO2+H2SO4=BaSO4��+H2O2����4��Cu��ϡ�����Ӧ��������������Ե�H2O2������������ͭ��ˮ����Ӧ�����ӷ���ʽΪCu+2H++H2O2=Cu2++2H2O��
�ʴ�Ϊ��Cu+2H++H2O2=Cu2++2H2O��
��5��NaH+H2O=NaOH+H2��
1mol 1mol 1mol
2NaOH+2Al+2H2O=2NaAlO2+3H2����
1mol 1mol 1.5mol
n��H2��=1mol+1.5mol=2.5mol��
V��H2��=2.5mol��22.4L/mol=56L��
�ʴ�Ϊ��56L��
��6��D��ij������ʵ���ɫ��ΪNa2O2�����Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ����������غ��֪������ΪFe��OH��3��NaCl��FeCl3��
��Ӧ�����ӷ���ʽΪ3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+��
�ʴ�Ϊ��3Na2O2+6Fe2++6H2O=4Fe��OH��3��+6Na++2Fe3+��
���������⿼��Ԫ�ص��ƶϺ�Ԫ�ػ�����֪ʶ����Ŀ�Ѷ��еȣ���������ڳ���������Ĵ���Ϊͻ�ƿڣ�ע����Ԫ�ػ�����Ŀ��飬����ʱע����������Ϣ��

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ