��Ŀ����
9��һ��������������ԭ��Ӧ����ʽ���Բ�д���������뷴Ӧʽ����һ���ǡ�������Ӧ��ʽ��һ���ǡ���ԭ��Ӧ��ʽ����2Fe3++Cu�T2Fe2++Cu2+�IJ�д����ǣ�������ӦΪ��Cu-2e-�TCu2+����ԭ��ӦΪ��2Fe3++2e-�T2Fe2+����1������������Ϣ����Ӧ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���Ϊ�������뷴Ӧʽ����
��������Ӧ��3Cu-6e-=3Cu2+��
�ڻ�ԭ��Ӧ��8H++2NO3-+6e-=4H2O+2NO����
��2����֪����ȼ�ϵ�صİ뷴Ӧʽ�ֱ�Ϊ��CH4+10OH--8e-�TCO32-+7H2O��O2+2H2O+4e-�T4OH-�����ܷ�ӦΪCH4+2OH-+2O2�TCO32-+3H2O��
���� ��1��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��Ӧ�У�Cuʧ���ӷ���������Ӧ��NO3-�õ��ӷ�����ԭ��Ӧ���ݴ˷�����
��2��ԭ������������缫����ʽ��Ӽ��ɵõ��ܷ�Ӧ��
��� �⣺��1��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��Ӧ�У�Cuʧ���ӷ���������Ӧ��NO3-�õ��ӷ�����ԭ��Ӧ��
��������Ӧ��3Cu-6e-=3Cu2+���ʴ�Ϊ��3Cu-6e-=3Cu2+��
�ڻ�ԭ��Ӧ��8H++2NO3-+6e-=4H2O+2NO�����ʴ�Ϊ��8H++2NO3-+6e-=4H2O+2NO����
��2����֪����ȼ�ϵ�صİ뷴Ӧʽ�ֱ�Ϊ��CH4+10OH--8e-�TCO32-+7H2O��2O2+4H2O+8e-�T8OH-��ԭ������������缫����ʽ��Ӽ��ɵõ��ܷ�Ӧ�����ܷ�Ӧʽ��CH4+2OH-+2O2�TCO32-+3H2O���ʴ�Ϊ��CH4+2OH-+2O2�TCO32-+3H2O��
���� ���⿼����������ԭ��Ӧ��ԭ��ص缫��Ӧ����д����Ŀ�ѶȲ������ڶԻ���֪ʶ�Ŀ��飮

��ϰ��ϵ�д�
�����Ŀ
19�����������У����ڴ�������ǣ�������
| A�� | �� | B�� | �´� | C�� | ʳ�õ����� | D�� | ���� |
20�����������ܹ�ʢ�ŵ������ǣ�������
| A�� | ϡ���� | B�� | Ũ���� | C�� | NaOH��Һ | D�� | ϡ���� |
17����д��ȥ���������е��������ʣ�������Ϊ���ʣ������õ��Լ��Ͳ�������
| ��� | �������� | �����Լ� | �������� |
| �� | ��ȩ�����ᣩ | NaOH��Һ | |
| �� | CH3CH2OH��ˮ�� | ||
| �� | �������ӣ� | ||
| �� | �������������ᣩ |
4������ʯ�͵ķ�������У��е���ߵ��ǣ�������
| A�� | ���� | B�� | ú�� | C�� | ���� | D�� | ���� |
14����NA��������٤��������Щ��˵����ȷ���ǣ�������
| A�� | 42g C3H6�к��еĹ��õ��Ӷ���ĿΪ8NA | |
| B�� | 2g CaCO3��8g KHCO3��ɵĻ������̼ԭ����Ϊ0.1 NA | |
| C�� | ��״���£�22.4L������22.4L��������ԭ������Ϊ2NA | |
| D�� | 1L 0.01mol•L-1 KAl��SO4��2��Һ�к��е���������ĿΪ0.02 NA |
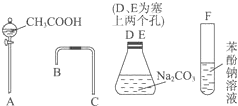 ���һ����һ�������ʵ���װ��ͼ����֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH��
���һ����һ�������ʵ���װ��ͼ����֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH�� ��
��