��Ŀ����
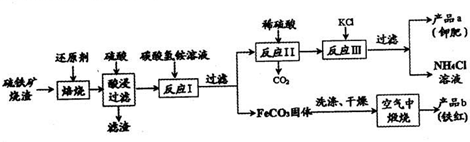
��������������Ҫ�ɷ�Fe2O3��Fe3O4��FeO��SiO2�ȣ��ǹ�ҵ��������ķ��������������������Ʊ�����Ȳ�Ʒ����������ͼ��ʾ��

��1����������������ʱ�����ӵĻ�ԭ�����п��ܵ��� ��������ĸ��
A���� B��п C��̼
��2����������˺���Һ�е���Ҫ�ɷ��� ��
��3����Ӧ��ķ�Ӧ�¶�һ���������35�����£���Ŀ���� ��
��4������������FeCO3���ɲ�Ʒb�Ļ�ѧ��Ӧ����ʽΪ ��
��5�������Ʒa���Ƿ����Ȼ������ʵ�ʵ������ǣ�ȡ������Ʒa���Թ��������Һ�� ��
���𰸡�
��10�֡�
��1��C��2�֣� ��2��FeSO4 ����H2SO4��FeSO4����2�֣�
��3����ֹNH4HCO3�ֽ⣨�����Fe2����ˮ�⣩��2�֣�
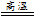 ��4��4FeCO3 + O2
2Fe2O3 + 4CO2��2�֣�
��4��4FeCO3 + O2
2Fe2O3 + 4CO2��2�֣�
��5���μӹ���Ba(NO3)2��Һ�����˺�����Һ�еμ�AgNO3��Һ��2�֣�

��ϰ��ϵ�д�
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д�
�����Ŀ

��װ��A�п���ѡ�������Լ��е�____����д�� ����ĸ����
a��(NH4)2SO4����ʯ�� b��NH4Cl��Ca(OH)2���� c��NH4HCO3���� d��NH4Cl����
��װ��C���Լ����ѡ��_________����д�Լ����ƣ���
���ռ�NH3ʱ������Ӧ��_____����д���ܿڴ��ţ�ͨ�롣
(4)д����ˮ��NH4HCO3��Һ��Ӧ�����ӷ���ʽ_______________________
a��(NH4)2SO4����ʯ�� b��NH4Cl��Ca(OH)2���� c��NH4HCO3���� d��NH4Cl����
��װ��C���Լ����ѡ��_________����д�Լ����ƣ���
���ռ�NH3ʱ������Ӧ��_____����д���ܿڴ��ţ�ͨ�롣
(4)д����ˮ��NH4HCO3��Һ��Ӧ�����ӷ���ʽ_______________________