��Ŀ����
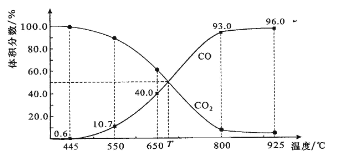
����Ŀ����1����һ���¶��£��ݻ�Ϊ0.5L���ܱ������У���һ�����Ķ��������Ͷ������������Ϻ�����Ӧ��SO2(g)��NO2(g) ![]() NO(g) ��SO3(g)������ƽ����ϵ��ͨ������O2��ƽ�� ________�ƶ�������ҡ���������������NO��Ũ�Ƚ�_______������������١����䡱����c��NO����c��NO2��֮�� ________������������١����䡱����
NO(g) ��SO3(g)������ƽ����ϵ��ͨ������O2��ƽ�� ________�ƶ�������ҡ���������������NO��Ũ�Ƚ�_______������������١����䡱����c��NO����c��NO2��֮�� ________������������١����䡱����
��2����̼�����Һ���ɵõ��Ĺ���������____________ ��ԭ����______________________��
��̼���Ⱶ��Һ���ɵõ��Ĺ���������____________ ��ԭ����______________________��
��3���������ӷ���ʽ��ʾ��ĭ��������ɷ֣�NaHCO3��Al2(SO4)3�������ԭ����___________��
��4��25 ��ʱ����a mol��L-1��ˮ��0.01 mol��L-1����������ϣ���Ӧƽ��ʱ��Һ��c(NH)��c(Cl��)������Һ��________(��ᡱ��������С�)�ԡ��ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��________��
��5����������ȡ0.2 mol��L-1MOH��Һ��0.1 mol��L-1����������ϣ���û����Һ��pH < 7��˵��MOH�ĵ���̶�___________(�>������<����=��)MCl��ˮ��̶ȡ���Һ�и�����Ũ���ɴ�С��˳��Ϊ______________________��
���𰸡����� ��С ���� K2CO3 ���ܼ��ȹ��̴ٽ���K2CO3��ˮ�⣬�����ɵ�KHCO3��KOH��Ӧ������K2CO3 BaCO3 ��������Һ��Ba(HCO3)2�ֽ�����BaCO3��CO2��H2O������Ũ���IJ��Ͻ��У�CO2�����ݳ������ʣ��BaCO3 Al3����3HCO===Al(OH)3����3CO2�� �� ![]() < c(Cl��) > c(M+) > c(H+) > c(OH��)
< c(Cl��) > c(M+) > c(H+) > c(OH��)
��������
(1)��SO2(g)��NO2(g) ![]() NO(g) ��SO3(g)ƽ����ϵ��ͨ������O2��NO��O2��Ӧ����NO2���ݴ��жϣ�(2)̼����е�̼�������ˮ�⣬�Ե�һ��ˮ��Ϊ����̼���Ⱶ����Һ���������ֽ⣬�ݴ��жϣ�(3)Al2(SO4)3��Һ��NaHCO3��Һ����˫ˮ�ⷴӦ���ݴ���д����ʽ��(4)���ݵ���غ��жϣ�NH3H2O�ĵ��볣��Kb=
NO(g) ��SO3(g)ƽ����ϵ��ͨ������O2��NO��O2��Ӧ����NO2���ݴ��жϣ�(2)̼����е�̼�������ˮ�⣬�Ե�һ��ˮ��Ϊ����̼���Ⱶ����Һ���������ֽ⣬�ݴ��жϣ�(3)Al2(SO4)3��Һ��NaHCO3��Һ����˫ˮ�ⷴӦ���ݴ���д����ʽ��(4)���ݵ���غ��жϣ�NH3H2O�ĵ��볣��Kb=![]() ����ϵ��볣�����㣻(5)�����£�0.2molL-1MOH��Һ��0.1molL-1HCl��Һ�������ϣ���Һ�е�������MCl��MOH����û����Һ��pH��7��˵����ĵ���̶�С���ε�ˮ��̶ȣ����ݵ���غ��ж�����Ũ�ȴ�С��
����ϵ��볣�����㣻(5)�����£�0.2molL-1MOH��Һ��0.1molL-1HCl��Һ�������ϣ���Һ�е�������MCl��MOH����û����Һ��pH��7��˵����ĵ���̶�С���ε�ˮ��̶ȣ����ݵ���غ��ж�����Ũ�ȴ�С��
(1)��SO2(g)��NO2(g) ![]() NO(g) ��SO3(g)ƽ����ϵ��ͨ������O2��NO��O2��Ӧ����NO2����ϵ��NOŨ�Ƚ��ͣ�NO2Ũ������ʹ��ѧƽ�������ƶ�������NԪ���غ㣬��c(NO)��c(NO2)֮�Ͳ��䣬�ʴ�Ϊ�����ң����٣����䣻
NO(g) ��SO3(g)ƽ����ϵ��ͨ������O2��NO��O2��Ӧ����NO2����ϵ��NOŨ�Ƚ��ͣ�NO2Ũ������ʹ��ѧƽ�������ƶ�������NԪ���غ㣬��c(NO)��c(NO2)֮�Ͳ��䣬�ʴ�Ϊ�����ң����٣����䣻
(2)��̼����е�̼�������ˮ�⣬���ܼ��ȹ����ܴٽ�̼���ˮ�⣬�����ɵ�̼����غ��������ط�Ӧ������̼��أ��ʴ�Ϊ��̼��أ����ܼ��ȹ����ܴٽ�̼���ˮ�⣬�����ɵ�̼����غ��������ط�Ӧ������̼��أ�
��̼���Ⱶ����Һ�����ȷֽ�����̼�ᱵ��ˮ�Ͷ�����̼������Ũ���IJ��Ͻ��У�CO2�����ݳ������ʣ��BaCO3���������õ��Ĺ���������̼�ᱵ���ʴ�Ϊ��̼�ᱵ����������Һ��Ba(HCO3)2�ֽ�����BaCO3��CO2��H2O������Ũ���IJ��Ͻ��У�CO2�����ݳ������ʣ��BaCO3��
(3)Al2(SO4)3��Һˮ������ԣ�NaHCO3��Һˮ��ʼ��ԣ�������ٽ�ˮ������Al(OH)3��CO2����Ӧ�����ӷ���ʽΪAl3++3HCO3-=Al(OH)3��+3CO2�����ʴ�Ϊ��Al3++3HCO3-=Al(OH)3��+3CO2����
(4)���ݵ���غ�ɵã�c(H+)+c(NH4+)=c(Cl-)+c(OH-)����֪c(NH4+)=c(Cl-)������c(H+)=c(OH-)��������Һ�����ԣ����볣��ֻ���¶��йأ����ʱNH3H2O�ĵ��볣��Kb=![]() =
=![]() =
=![]() molL-1���ʴ�Ϊ���У�
molL-1���ʴ�Ϊ���У�![]() molL-1����λ�ɲ�д����
molL-1����λ�ɲ�д����
(5)������ȡ0.2molL-1MOH��Һ��0.1molL-1HCl��Һ�������ϣ���Һ�е�������MCl��MOH����û����Һ��pH��7��˵����ĵ���̶�С���ε�ˮ��̶ȣ���Һ�����ԣ���c(H+)��c(OH-)����ϵ���غ�֪c(Cl-)��c(M+)��������������Ũ�ȴ��������Ӻ�����������Ũ�ȣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊc(Cl-)��c(M+)��c(H+)��c(OH-)���ʴ�Ϊ������c(Cl-)��c(M+)��c(H+)��c(OH-)��

����Ŀ��2SO2(g)+ O2(g) ![]() 2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
�� | �� | �� | ||
��ʼ���ʵ��� | n(SO2)/mol | 0.4 | 0.8 | 0.8 |
n(O2)/mol | 0.24 | 0.24 | 0.48 | |
SO2��ƽ��ת����/% | 80 | ��1 | ��2 |
A. ���з�Ӧ��ƽ�ⳣ��С����
B. ���¶��£�ƽ�ⳣ��ֵΪ400
C. ƽ��ʱ������c(SO3)�Ǽ��е�2��
D. ƽ��ʱ������O2��ת���ʴ�������O2��ת����