��Ŀ����
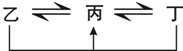 ��A��B��C��D��E��F����ǰ�����ڵ�Ԫ�أ�ԭ��������������A��B��C��D��E��Ϊ������Ԫ�أ�D��FԪ�ض�Ӧ�ĵ���Ϊ�ճ������г���������Aԭ�Ӻ���ֻ��һ�����ӣ�Ԫ��A��B�γɵ���̬���������10e-���ռ乹��Ϊ�����Σ�CԪ��ԭ�ӵ�����������������Ӳ�����3����C��Eͬ���壮ͼ�о���D��FԪ�ص����ʾ�����ͼʾת����ϵ��
��A��B��C��D��E��F����ǰ�����ڵ�Ԫ�أ�ԭ��������������A��B��C��D��E��Ϊ������Ԫ�أ�D��FԪ�ض�Ӧ�ĵ���Ϊ�ճ������г���������Aԭ�Ӻ���ֻ��һ�����ӣ�Ԫ��A��B�γɵ���̬���������10e-���ռ乹��Ϊ�����Σ�CԪ��ԭ�ӵ�����������������Ӳ�����3����C��Eͬ���壮ͼ�о���D��FԪ�ص����ʾ�����ͼʾת����ϵ���پ���DԪ�ص��ҡ������������ת��ȫΪ��������ԭ��Ӧ��
�ھ���FԪ�ص��ң����ʣ��������������ת��ȫΪ������ԭ��Ӧ��
��ش��������⣺
��1��������ĵ���ʽΪ
��2��FԪ�������ڱ��е�λ��
��3��������DԪ�ص����붡����Һ�з�����Ӧ�����ӷ���ʽ
��4���������ֱ��Ǻ�FԪ�صļ������ӣ����麬�������������ӵĻ����Һ�еĵͼ����ӣ�����������KMnO4��Һ�����Ӧ�����ӷ���ʽΪ��
��5����֪�����»�����FE��Ksp=6��10-18 mol2?L-2�������½�1.0��10-5mol?L-1��Na2E��Һ�뺬FSO4��Һ�������3��2��ϣ����г���FE���ɣ��������FSO4��Ũ��Ҫ��
������A��B��C��D��E��F����ǰ�����ڵ�Ԫ�أ�ԭ��������������A��B��C��D��E��Ϊ������Ԫ�أ�D��FԪ�ض�Ӧ�ĵ���Ϊ�ճ������г���������AԪ��ԭ�Ӻ���ֻ��һ�����ӣ���AΪHԪ�أ�CԪ��ԭ�ӵ�����������������Ӳ�����3������Cԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6����CΪO��Ԫ�أ�E��Cͬ���壬��EΪSԪ�أ�Ԫ��A��B�γɵ���̬���������10e-���ռ乹��Ϊ�����Σ���BΪNԪ�أ���ΪNH3�� D��FΪ��������Ԫ�أ�������DԪ�ص��ҡ������������ת��ȫΪ��������ԭ��Ӧ��DΪAlԪ�أ����ΪAl��OH��3���ҡ����ֱ�ΪAlO2-��Al3+�е�һ�֣�������FԪ�ص��ҡ������������ת��ȫΪ������ԭ��Ӧ��FԪ��Ϊ��������Ԫ�أ���FΪFeԪ�أ���ΪFe���ʡ���ΪFe2+����ΪFe3+���ݴ˽��
����⣺A��B��C��D��E��F����ǰ�����ڵ�Ԫ�أ�ԭ��������������A��B��C��D��E��Ϊ������Ԫ�أ�D��FԪ�ض�Ӧ�ĵ���Ϊ�ճ������г���������AԪ��ԭ�Ӻ���ֻ��һ�����ӣ���AΪHԪ�أ�CԪ��ԭ�ӵ�����������������Ӳ�����3������Cԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6����CΪO��Ԫ�أ�E��Cͬ���壬��EΪSԪ�أ�Ԫ��A��B�γɵ���̬���������10e-���ռ乹��Ϊ�����Σ���BΪNԪ�أ���ΪNH3�� D��FΪ��������Ԫ�أ�������DԪ�ص��ҡ������������ת��ȫΪ��������ԭ��Ӧ��DΪAlԪ�أ����ΪAl��OH��3���ҡ����ֱ�ΪAlO2-��Al3+�е�һ�֣�������FԪ�ص��ҡ������������ת��ȫΪ������ԭ��Ӧ��FԪ��Ϊ��������Ԫ�أ���FΪFeԪ�أ���ΪFe���ʡ���ΪFe2+����ΪFe3+��
��1���������ΪNH3�������ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��FΪFeԪ�أ�λ�����ڱ��е������ڵڢ��壻�ǽ�����O��S�����⻯���ȶ���H2O����H2S��
�ʴ�Ϊ���������ڵڢ��壻���ڣ�
��3���ҡ����ֱ�ΪAlO2-��Al3+�е�һ�֣����߷�Ӧ���ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3����
�ʴ�Ϊ��Al3++3AlO2-+6H2O=4Al��OH��3����
��4���������Ӿ��л�ԭ�ԣ������Ը�������������������ӣ����Ӧ�����ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
��5�������»�����FeS��Ksp=6��10-18 mol2?L-2�������½�1.0��10-5mol?L-1��Na2S��Һ�뺬FeSO4��Һ�������3��2��ϣ���Ϻ���Һ��c��S2-��=
mol/L=6.0��10-6mol/L�����г���FeS���ɣ�������Һ��c��Fe2+����
mol/L=10-12mol/L��������c��FeSO4����
mol/L=2.5��10-12 mol/L��
�ʴ�Ϊ�����ڻ����2.5��10-12 mol/L��
��1���������ΪNH3�������ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��FΪFeԪ�أ�λ�����ڱ��е������ڵڢ��壻�ǽ�����O��S�����⻯���ȶ���H2O����H2S��
�ʴ�Ϊ���������ڵڢ��壻���ڣ�
��3���ҡ����ֱ�ΪAlO2-��Al3+�е�һ�֣����߷�Ӧ���ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3����
�ʴ�Ϊ��Al3++3AlO2-+6H2O=4Al��OH��3����
��4���������Ӿ��л�ԭ�ԣ������Ը�������������������ӣ����Ӧ�����ӷ���ʽΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
��5�������»�����FeS��Ksp=6��10-18 mol2?L-2�������½�1.0��10-5mol?L-1��Na2S��Һ�뺬FeSO4��Һ�������3��2��ϣ���Ϻ���Һ��c��S2-��=
| 1.0��10-5��3 |
| 3+2 |
| 6��10-18 |
| 6��10-6 |
| 10-12��5 |
| 2 |
�ʴ�Ϊ�����ڻ����2.5��10-12 mol/L��
���������⿼��Ԫ�ؼ�������ƶϡ����û�ѧ���Ԫ�ػ���������ʡ��ܶȻ��йؼ���ȣ��ѶȽϴ��ض�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����

��ϰ��ϵ�д�
�����Ŀ





