��Ŀ����
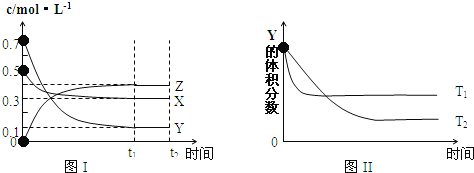
����Ŀ���������������ݻ����ȵĺ����ܱ������У�������0.lmolCO��0.2molH2���ڴ����������·�����Ӧ��CO(g)+2H2(g) ![]() CH3OH(g)���������������ƽ��������CH3OH������������¶ȵı仯��ͼ��ʾ��
CH3OH(g)���������������ƽ��������CH3OH������������¶ȵı仯��ͼ��ʾ��
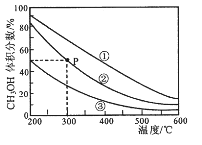
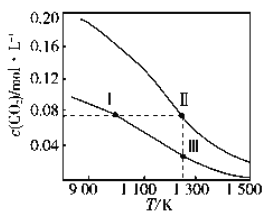
����˵����ȷ����
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. ���������ݻ�����>��>��
C. ��P�㣬COת����Ϊ75%
D. ��P�㣬�����������ٳ���CO��H2 �� CH3OH �� 0.025mol����ʱ v(CO)��<v (CO)��
���𰸡�AC
��������A����ѡһ������������¶ȣ��״������������С��ƽ�������ƶ���˵���淴ӦΪ���ȷ�Ӧ������ӦΪ���ȷ�Ӧ��A��ȷ��B����ͬ�¶��� ����ѹǿ��ƽ�������ƶ����״����������Խ��>��>�ۣ�˵�����е�ѹǿ����������С���������СΪ�٣��ڣ��ۣ�B����C��P��״����������Ϊ50%����COת�������ʵ���Ϊx,������ת����2x,�״�������x,50%=x/(0.1-x+0.2-2x+x)�õ�x =0.075������CO��ת����Ϊ0.075/0.1*100%=75% ��C��ȷ��D������������ΪV��P��ƽ���ʱ��CH3OH�����ʵ���Ϊ0.075mol��Ũ��Ϊ��0.075/V��mol��L-1��CO�����ʵ���Ϊ0.025mol��Ũ��Ϊ��0.025/V��mol��L-1����H2�����ʵ���Ϊ0.05mol��Ũ��Ϊ��0.05/V��mol��L-1��K=��0.075/V��/��0.025/V��*��0.05/V��2=3*104*V2/25���ٳ���CO��H2 �� CH3OH �� 0.025mol��ͬ���ķ�������õ�Ũ����Q=2*104*V2/7.52��Q��K������v(CO)����v (CO)����D������ȷ��ΪAC

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣���ش��й����⣺
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ ��
��2���������γ��������������Ԫ��������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ ��
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������Ϊ���û�ѧʽ��ʾ����
��4����Ԫ�����Ԫ�����ߺ˵����֮���� ��