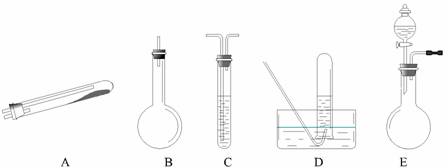
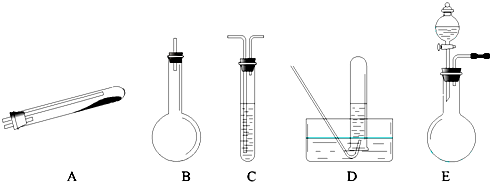
��Ŀ����
ʵ���ҿ���ͨ������;������ȡ������
�ټ�����������NH3��ԭCuO�Ƶô�����N2�ͻ���ͭ�ۣ���Ӧ�Ļ�ѧ����ʽΪ��
![]()
�� ������ͨ�����ȵĻ���ͭ���Ƶýϴ�����N2
�� ����NaNO2���ж��ԣ���NH4Cl�Ļ��Ũ��Һ��ȡN2����Ӧ�Ļ�ѧ����ʽΪ��
![]()
������;����N2�ɹ�ѡ���ʵ����������ͼ��ʾ��������Ҫ������������̨�����С���Ȧ��ʯ�������ƾ��Ƶ�δ�г���

��l����;�� �� ��ȡN2ʱ����İ���Ҫ��Ũ��ˮ����ʯ����ԭ����ȡ����ѧ����ʽΪ��
![]() �����Ҫ������������ �ģ���������ĸ����ͬ����Ϊ��������װ�á�Ҫ��ȡ���ռ�������N2��������������ˮ����������Ӧʹ�õ����������е�
�����Ҫ������������ �ģ���������ĸ����ͬ����Ϊ��������װ�á�Ҫ��ȡ���ռ�������N2��������������ˮ����������Ӧʹ�õ����������е�
��2�� �� �� �� ����;��������Эͬʹ�á����ַ�����;�� �� ����ŵ���
��3����� E װ�������Եķ����� ������ E װ�û�������ȡ�������� ��д�����֣���
( l ) E A ��D��A��C��D
( 2 ) Cu �� CuO ����ѭ��ʹ�ã�������ʹ���ж����ʡ������������𰸾��ɵ÷֣�
( 3 ����ֹˮ�мн� E ���齺�ܣ����Һ©���м�������ˮ��������������Һ�汣�ֺ㶨����֤����װ�ò�©��������������Ҳ���֣��� H2��O2��CO2��C12��SO2��NO2�� NO�ȣ�д������ 3 �ֵ� 2 �֣�д�� 2 �ֵ� l �֣�д�� l �ֵ� 0 �֣�������ÿ�� 2 �֣��� 10 �֣�
����:
����Ľ���˼·����ʹƷ����Һ��ɫ��������C12��SO2�����������֪C12 ��FeCl3������Һ�����ˮ���Ƶú��ɫFe��OH��3����

 N2+3Cu+3H2O
N2+3Cu+3H2O