��Ŀ����
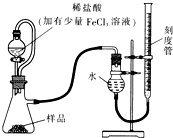 ������þ��MgO2��������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г����������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����
������þ��MgO2��������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г����������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�������1��ij�о�С��������ͼװ�òⶨһ����������Ʒ�й�����þ�ĺ�����
��ʵ��ǰ����еIJ�����
���ú�ѹ��Һ©�����ŵ��У�ʹ��Һ©���е���Һ˳�����£�
��ʵ������ʱ�����ָ������£���
��2��ʵ���һ���ͨ���������ַ����ⶨ��Ʒ�й�����þ�ĺ�����
������ȡa g��Ʒ����������ϡ���ᣬ��ַ�Ӧ���ټ��� NaOH��Һ��Mg2+������ȫ�����ˡ�ϴ�Ӻ�����������գ����յõ�b g���壮
������ȡ0.1g��Ʒ���ڵ���ƿ�У�����15mL0.6mol/LKI��Һ���������ᣬҡ�Ⱥ��ڰ�������5min��Ȼ����0.1mol/L Na2S2O3��Һ�ζ����ζ����յ�ʱ������VmL Na2S2O3��Һ������֪��I2+2Na2S2O3=Na2S4O6+2NaI��
����֪������Ksp[Mg��OH��2]=1��10-11��Ϊʹ����I��Mg2+��ȫ����[����Һ��c��Mg2+����1��10-5mol/L]����Һ��pH����Ӧ����
�ڷ������еζ�ǰ���������
��������1����ʵ��ǰ����еIJ����Ǽ��װ�õ������ԣ�ͨ������˫��ˮ�ֽ����ɵ��������ɼ�������þ�ĺ��������Ȼ������������ӿ�˫��ˮ�ķֽ⣬�ӿ췴Ӧ���ʣ�
�������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
��2���ٸ���������þ���ܶȻ����������Һ�����������ӵ�Ũ�ȣ������Һ��PH������b������þ������a�ǹ�����þ������þ��������������������ʵ�������ʽ������������ʵ����������ݹ�����þ�����ʵ��������������������
�����ڵ�����������ɫ����������������Һ��ָʾ�������ݵ����غ��ҳ���ϵʽ�������������þ������������
�������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
��2���ٸ���������þ���ܶȻ����������Һ�����������ӵ�Ũ�ȣ������Һ��PH������b������þ������a�ǹ�����þ������þ��������������������ʵ�������ʽ������������ʵ����������ݹ�����þ�����ʵ��������������������
�����ڵ�����������ɫ����������������Һ��ָʾ�������ݵ����غ��ҳ���ϵʽ�������������þ������������
����⣺��1����ʵ��װ�����Ӻ��Ժ�ʵ��ǰ����еIJ����Ǽ��װ�õ������ԣ�������þ����ˮ����˫��ˮ��˫��ˮ�ֽ⣬ͨ������˫��ˮ�ֽ����ɵ��������ɼ�������þ�ĺ��������Ȼ������������ӿ�˫��ˮ�ķֽ⣬�ӿ췴Ӧ���ʣ�
�ʴ�Ϊ�����װ�õ������ԣ��������������H2O2�ķֽ⣩��
�����������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
�ʴ�Ϊ������������Һ��������������������Ӱ�죻
����������������ѹǿӰ��������ڶ���֮ǰ��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
�ʴ�Ϊ�����Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��
��2���ٸ���������þ���ܶȻ�������֪������Һ��c��Mg2+��=l��10-5mol/Lʱ��Ksp[Mg��OH��2]=1��10-11=c��Mg2+��?c2��OH-������Һ��OH-Ũ�ȵ���1��10-3mol/L��������Һ��pH=11��
�������þ�����ʵ�����xmol������þ���ʵ�����ymol����56x+40y=a����x+y����40=b�����x=
������I�й�����þ����������Ϊ��
=
��
�ʴ��ǣ�11��
��
�ڷ���������þ���������ԣ��ܰѵ⻯���������ɵ��ʵ⣬Ȼ��������������Ƶζ����ɵĵ��ʵ⼴�ɣ����ڵ�����������ɫ����������������Һ��ָʾ����
���ݷ�Ӧ����ʽ��I2+2Na2S2O3=Na2S4O6+2NaI�����ݵ����غ��ҳ���ϵʽ��MgO2��I2������n��MgO2��=n��I2��=
n��Na2S2O3��=0.1mol/L��V��10-3L=5V��10-5mol��
������þ�����������ǣ�
��100%=2.8V%���ʴ�Ϊ��������Һ��2.8V%��
�ʴ�Ϊ�����װ�õ������ԣ��������������H2O2�ķֽ⣩��
�����������������¶Ⱥ�ѹǿӰ��������ú�ѹ��Һ©�����ŵ㻹������������Һ��������������������Ӱ�죬ʹ��Һ©���е���Һ˳�����£�
�ʴ�Ϊ������������Һ��������������������Ӱ�죻
����������������ѹǿӰ��������ڶ���֮ǰ��Ӧ�ý��Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��Ȼ����ƽ�ӿ̶��߶�����
�ʴ�Ϊ�����Ҳ�̶ȹܻ��������ƶ�ֱ������Һ����ƽ��
��2���ٸ���������þ���ܶȻ�������֪������Һ��c��Mg2+��=l��10-5mol/Lʱ��Ksp[Mg��OH��2]=1��10-11=c��Mg2+��?c2��OH-������Һ��OH-Ũ�ȵ���1��10-3mol/L��������Һ��pH=11��
�������þ�����ʵ�����xmol������þ���ʵ�����ymol����56x+40y=a����x+y����40=b�����x=
| a-b |
| 16 |
| ||
| a |
| 7(a-b) |
| 2a |
�ʴ��ǣ�11��
| 7(a-b) |
| 2a |
�ڷ���������þ���������ԣ��ܰѵ⻯���������ɵ��ʵ⣬Ȼ��������������Ƶζ����ɵĵ��ʵ⼴�ɣ����ڵ�����������ɫ����������������Һ��ָʾ����
���ݷ�Ӧ����ʽ��I2+2Na2S2O3=Na2S4O6+2NaI�����ݵ����غ��ҳ���ϵʽ��MgO2��I2������n��MgO2��=n��I2��=
| 1 |
| 2 |
������þ�����������ǣ�
| 5V��10-5��56 |
| 0.1 |
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
�����Ŀ



 ������þ��MgO2��������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г����������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����
������þ��MgO2��������ϡ�ᣬ�����������������⣬��ҽѧ�Ͽ���Ϊ������ȣ�������þ��Ʒ�г����������MgO��ʵ���ҿ�ͨ�����ַ����ⶨ��Ʒ�й�����þ�ĺ�����