��Ŀ����
(15��)��ˮ����Ԫ����Br-��ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ�����������ͼ��
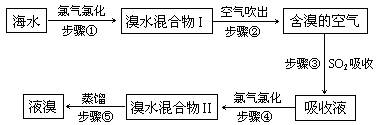
(1)����ٷ�Ӧ�����ӷ���ʽ�� ��
(2)����۷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)Br��ԭ�������� �������ڱ���λ�� ���� �塣
(4)���������Ĺ����У��¶�Ӧ������80~90�档�¶ȹ�����Ͷ������������������ԭ�� ��
(5)Ϊʲô��ֱ���á���ˮ�����I����Ҫ�á���ˮ�����II���������õ�Һ��?
��

(1)����ٷ�Ӧ�����ӷ���ʽ�� ��
(2)����۷�Ӧ�Ļ�ѧ����ʽ�� ��
(3)Br��ԭ�������� �������ڱ���λ�� ���� �塣
(4)���������Ĺ����У��¶�Ӧ������80~90�档�¶ȹ�����Ͷ������������������ԭ�� ��
(5)Ϊʲô��ֱ���á���ˮ�����I����Ҫ�á���ˮ�����II���������õ�Һ��?
��
�ο��𰸹������£�
(1) 2Br- + Cl2�T�TBr2 + 2Cl- (3��)
(2) SO2 + Br2 + H2O�T�T2HBr + H2SO4 (3��)
(3)35 ���� ��VIIA (3��)
(4)�¶ȹ��ߣ�����ˮ���������ų���������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ͡�(3��)
(5)����ˮ�����I������Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ�����������������ɱ��ߣ�����������������SO2���ա��Ȼ����Ȳ���ʵ�����ǽ���ˮŨ���ˡ�(3��)
(1) 2Br- + Cl2�T�TBr2 + 2Cl- (3��)
(2) SO2 + Br2 + H2O�T�T2HBr + H2SO4 (3��)
(3)35 ���� ��VIIA (3��)
(4)�¶ȹ��ߣ�����ˮ���������ų���������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ͡�(3��)
(5)����ˮ�����I������Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ�����������������ɱ��ߣ�����������������SO2���ա��Ȼ����Ȳ���ʵ�����ǽ���ˮŨ���ˡ�(3��)
�����ѣ����������������ڹ�ҵ��ģ��ˮ����ij��÷���������һ�ֹ�������Ԥ�Ⱦ����ữ��Ũ����ˮ�У��������뺣ˮ�������ӷ�Ӧ��ʹ֮��Ϊ�����壺2Br-+Cl2�T�T2Cl-+Br2���̶�ͨ�������ˮ���������崵����������ʹ�����������ռ�SO2��������ת���������SO2+Br2+2H2O�T�TH2SO4+2HBr���Դﵽ������Ŀ�ġ�Ȼ�������������������õ���Ʒ�壺2HBr+Cl2�T�T2HCl+Br2���������õ�Һ�塣���ܿռ䣺����ⶼ��±�ص��ʣ���ˮ�����뺣ˮ���Ĺ�������������֮������Ҳ������ͬ����ˮ����ǽ�����ֲ���纣���������ɹ�ɡ����ճɺ���ң����ݺ�����γ�����Һ�����˵��������ú������ӵ���Һ��ͨ������������Ӧ��2I-+Cl2�T�T2Cl-+I2��������Ҫ�ⵥ�ʵ���Һ�������Ȼ�̼�������л��ܼ�������ȡ�õ�����л���Һ������ٷ���õ��ⵥ�ʡ�
Ӧ�Բ��ԣ��������߿��Ŀ�ͼ�ƶ����У��ص㿼���˻�ѧ����Դ�ۺ����á����������ȷ����֪ʶ���������ý�������ͺ�ˮ��Դ���¿γ̱��̲��еı������ݣ��ڸ߿�������Ҳ�������֡����������е�����ʯ��������������ݣ���ˮ��Դ��صĺ�ˮ���塢��ˮ��þ���Ǹ߿�������ȵ㣬ͬѧ��Ӧ�������ӡ�ֻ�����պ���ص���������ѧ�����Լ�����ʵ�鼼�ܾ���˳�����
Ӧ�Բ��ԣ��������߿��Ŀ�ͼ�ƶ����У��ص㿼���˻�ѧ����Դ�ۺ����á����������ȷ����֪ʶ���������ý�������ͺ�ˮ��Դ���¿γ̱��̲��еı������ݣ��ڸ߿�������Ҳ�������֡����������е�����ʯ��������������ݣ���ˮ��Դ��صĺ�ˮ���塢��ˮ��þ���Ǹ߿�������ȵ㣬ͬѧ��Ӧ�������ӡ�ֻ�����պ���ص���������ѧ�����Լ�����ʵ�鼼�ܾ���˳�����

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ