��Ŀ����
1��2-���������ڳ���������ɫҺ�壬�ܶ���2.18g/cm3���е���131.4�棬�۵���9.79�棬������ˮ�������ڴ�����ͪ���л��ܼ�����ʵ���ҿ�����ͼ��ʾװ�����Ʊ�1��2-�������飮�����Թ�c��װ��Ũ��ˮ��
��1��д���Ʊ�1��2-��������Ļ�ѧ����ʽ�� ���÷�Ӧ���� ��Ӧ���Ӧ���ͣ���
��2����ȫƿa���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�c�Ƿ����������������������a�е������ǣ� ��
��3������b��NaOH��Һ�������� ��
��4�����Թ�c������ˮ�е�Ŀ���ǣ� ��
��5������d��NaOH��Һ�������� ����������Ӧ�����ӷ���ʽΪ�� ��
��2����d����ʱ�����岻��ͨ������b�����������ѹǿ��ˮѹ��ֱ�������У�������������ܣ�
��3��b��ʢ��������Һ����������ϴ����ϩ��
��4���嵥���ӷ���
��5����������������������Һ���գ�
����⣺��1����ϩ����ļӳɵõ�1��2-�������飬�ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br���ӳɣ�
��2��ͨ���۲�ʵ��װ�ã���֪a�ǰ�ȫƿ��ֹҺ�嵹������ƿ��ˮ�в���һֱ�����ܣ���Ҫ�����Ǽ���Թ�c�Ƿ��������c����ʱ�����岻��ͨ������b�����������ѹǿ��ˮѹ��ֱ�������У�������������ܣ��ʴ�Ϊ��a�в�������ˮ������������ˮ�������
��3��b��ʢ��������Һ����������ϴ����ϩ����ȥ���к��е����ʣ�CO2��SO2�ȣ����ʴ�Ϊ����ȥ��ϩ�е�CO2��SO2��
��4�������ܼ�����Ļӷ����ʴ�Ϊ��������Ļӷ���
��5����������������������Һ���գ���Ӧ�ķ���ʽΪ��Br2+2OH-=Br-+BrO-+H2O���ʴ�Ϊ��������������Br2+2OH-=Br-+BrO-+H2O��
���������⿼�����������ȡ�����ʣ��ѶȲ���ע�����հ�ȫƿ��ֹҺ�嵹����ԭ����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣

��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
| �ܶ�/g��mL-1 | �е�/�� | �ܽ��� | |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
| �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��(4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
| A������ƿδ���T����������Һ | B��������ƿת����Һʱ������Һ�彦�� |
| C��δϴ���ܽ������ձ� | D������ʱ�����ӿ̶��� |

��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����="==" NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
| | �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
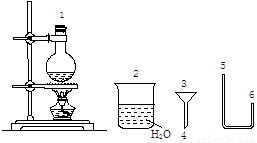
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
|
�ܶ�/g��mL-1 |
�е�/�� |
�ܽ��� |
|
������ |
1.461 |
38 |
������ˮ |
|
�Ҵ� |
0.789 |
78 |
������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
