��Ŀ����
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
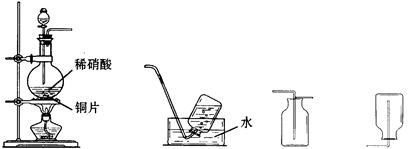
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
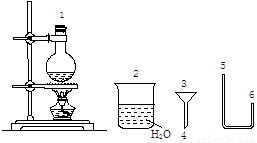
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
|
�ܶ�/g��mL-1 |
�е�/�� |
�ܽ��� |
|
������ |
1.461 |
38 |
������ˮ |
|
�Ҵ� |
0.789 |
78 |
������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
��1����� �����غ�ϸ�������� ��ά��ø����ø
��2��F �����ɲ�ͬ����̼�Dz�ͬ
��3����2������2��4���Թ��м��벻ͬ�����ĸ����أ�1�Ų��Ӹ�������Ϊ�հ���
��3������1��4���Թܷ�����ͬ�����˵Ļ�������һ��ʱ��
��4�����۲첢��¼��ϸ��������״��
����������

��19�֣�����������̵���Ҫ������ͳ��õ���������������ʵ������ģ�ҵ�������̿��Ʊ�������ص�����ͼ��
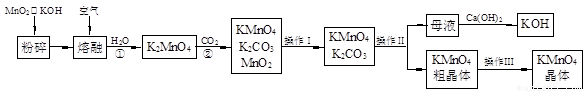
��1�������������Ϊ �������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ����ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe��������K2MnO4Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2���� MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԣ� ��
��4��KMnO4�����Խ����е�ǿ�����Թ㷺Ӧ���ڷ�����ѧ�С�
���磺2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O��ijͬѧ��KMnO4�ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȡ�����ȷ��ȡ6.3 g Na2SO3������Ʒ�����500 mL��Һ��ȡ25.00 mL������Һ������ƿ�У���0.01000 mol/L ������KMnO4��Һ���еζ����ζ�������±���ʾ��
|
�ζ�����[��Դ:][��Դ:Z&xx&k.Com] |
������Һ�����/mL[��Դ:ѧ#��#��Z#X#X#K] |
����Һ�����[��Դ:] |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 mL |
0.02 |
24.01 |
|
2 |
25.00 mL |
0.70 |
24.71 |
|
3 |
25.00 mL |
0.20 |
24.20 |
������500 mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ�������������ͷ�ιܡ�ҩ�� �� ��
���жϵζ��յ�������� ��
�����в����ᵼ�²ⶨ���ƫ�ߵ���
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿδ����
C���ζ�ǰ�ζ��ܼ��첿��������
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��������ʵ�����ݣ�����Na2SO3�Ĵ���Ϊ ��