��Ŀ����
��ˮ���зdz��ḻ�Ļ�ѧ��Դ���Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϣ��Իش��������⣺
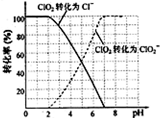
��1�������к���Ca2+��Mg2+��SO42-�����ʣ����ƺ�ɵñ���NaCl��Һ������ʱͨ������Һ�м����������ʣ������ʹ��Һ�����ԣ�
�ٹ�����Na2CO3��Һ�������ᡢ�۹�����NaOH��Һ���ܹ�����BaCl2��Һ�����˳����______
��2��д���������һ���Լ�����ܷ����Ļ�ѧ��Ӧ�����ӷ���ʽ______��
��1�������к���Ca2+��Mg2+��SO42-�����ʣ����ƺ�ɵñ���NaCl��Һ������ʱͨ������Һ�м����������ʣ������ʹ��Һ�����ԣ�
�ٹ�����Na2CO3��Һ�������ᡢ�۹�����NaOH��Һ���ܹ�����BaCl2��Һ�����˳����______
��2��д���������һ���Լ�����ܷ����Ļ�ѧ��Ӧ�����ӷ���ʽ______��
��1��SO42-��Ca2+��Mg2+�ȷֱ���BaCl2��Һ��Na2CO3��Һ��NaOH��Һ��Ӧ���ɳ���������ͨ�����˳�ȥ��Na2CO3��Һ�ܳ�ȥ������BaCl2��Һ�������ܳ�ȥ������Na2CO3��Һ��NaOH��Һ������Ӧ�ȼ�BaCl2��Һ�ټ�Na2CO3��Һ�����������ᣬ������ȷ˳��Ϊ���ܢۢ٢ڻ�ۢܢ٢ڣ�
�ʴ�Ϊ���ܢۢ٢ڻ�ۢܢ٢ڣ�
��2����NaOH��Na2CO3�������������ᷴӦ���������ӷ���ʽΪ��H++OH-�TH2O��2H++CO32-�TH2O+CO2����
�ʴ�Ϊ��H++OH-�TH2O��2H++CO32-�TH2O+CO2����
�ʴ�Ϊ���ܢۢ٢ڻ�ۢܢ٢ڣ�
��2����NaOH��Na2CO3�������������ᷴӦ���������ӷ���ʽΪ��H++OH-�TH2O��2H++CO32-�TH2O+CO2����
�ʴ�Ϊ��H++OH-�TH2O��2H++CO32-�TH2O+CO2����

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ