��Ŀ����
�Ȼ���ͭ(CuCl)Ϊ��ɫ��ĩ������ˮ���������л��ϳɴ��������������ϡ������ȹ�ҵ��ʵ���ҿɲ����������ƻ�ԭ�Ȼ�ͭ�ķ����Ʊ��Ȼ���ͭ����Ҫ������ͼ��ʾ��
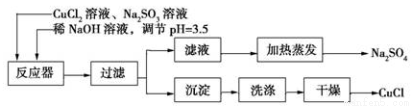
(1)���������Ʊ��Ȼ���ͭ�Ļ�ѧ����ʽΪ_________________ ��
��
(2)��ͼ�Ǽ���������Һ������Na2SO4��װ�á�װ��ͼ�д���һ�����ش��ô�����_____________��

(3)ϴ�Ӳ�Ʒ�Ȼ���ͭ�������Լ���___________����������Ƿ�ϴ�Ӹɾ��ķ�����_______________��
(4)CuCl��������Һ������ һ����̼�������Ȼ��ʻ���ͭ[Cu2Cl2(CO)2��2H2O]����ͼ����CO(����CO2����)��ԭCuO����֤�����������ʵ��װ��ͼ��
һ����̼�������Ȼ��ʻ���ͭ[Cu2Cl2(CO)2��2H2O]����ͼ����CO(����CO2����)��ԭCuO����֤�����������ʵ��װ��ͼ��
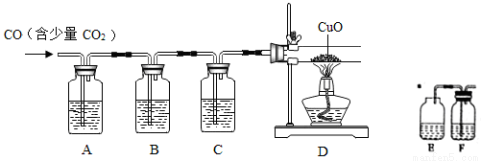
װ��A��ʢװ���DZ���NaOH��Һ����װ�õ�������_____________��װ��Bʢ�г����ʯ��ˮ������װ��B��������___________��ʢװCuCl��������Һ��װ����____________(ѡ��װ�����ṩ��װ����ĸ����)��
��ϰ��ϵ�д�
�����Ŀ
����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в���ȷ����( )
X | Y | Z |
| |
A | ������ | ������ | ������ | |
B | ǿ����� | ����� | ������ | |
C | ���� | ��ɢϵ | ����� | |
D | �û���Ӧ | ������ԭ��Ӧ | ���ӷ�Ӧ |


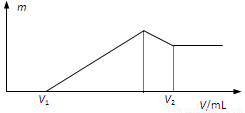
 OH��Һ�����У���ʹMg2+
OH��Һ�����У���ʹMg2+ Fe3O4(s)��4H2(g)��һ�ݻ��ɱ���ܱ������н��У����������ĸı���ʹ��Ӧ���ʼӿ����
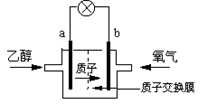
Fe3O4(s)��4H2(g)��һ�ݻ��ɱ���ܱ������н��У����������ĸı���ʹ��Ӧ���ʼӿ���� �����û����������ܼ�����200������ʱ���磬�Ҵ���رȼ״����Ч�ʸ߳�32���Ҹ���ȫ������ܷ�ӦΪ��C2H5OH +3O2=2CO2 +3H2O�����ʾ��ͼ���£�����˵������ȷ����
�����û����������ܼ�����200������ʱ���磬�Ҵ���رȼ״����Ч�ʸ߳�32���Ҹ���ȫ������ܷ�ӦΪ��C2H5OH +3O2=2CO2 +3H2O�����ʾ��ͼ���£�����˵������ȷ����