��Ŀ����
��10�֣�ѧϰ��ѧӦ����ȷ����������������������ȥ�������������������У����ǻ��������ָ����Ļ�ѧ���ʺͻ�ѧ��Ӧ��
��1����ҵ������������ˮ���������Ҫ�õ���ԭ���ǣ������ƣ� ����ҵ������Ҫ�豸��
��2������д����ҵ������������ʯ�ҷ�Ӧ��ȡƯ�۵Ļ�ѧ��Ӧ����ʽ�� ��
��3������д��θ��ƽ��������������������θ�ᣨ���ᣩ��������ӷ�Ӧ����ʽ�� ��
��4��ӡˢ��·�����ɸ߷��Ӳ��Ϻ�ͭ�����϶��ɣ�����ӡˢ��·��ʱ��Ҫ��FeCl3��Һ��Ϊ����ʴҺ����
��д��FeCl3��Һ����ʴ��ͭ�������ӷ���ʽ ��
��ij��ѧ��ȤС��Ϊ����ij���Ҹ�ʴ�Ժ����û����Һ��ɣ���̽�Ե���100 mL��ʴ��Ļ����Һ�м���2.80g���ۣ����ȫ���ܽ���δ������������������Һ�����Ϊ����д��ѧʽ����
��1����ҵ������������ˮ���������Ҫ�õ���ԭ���ǣ������ƣ� ����ҵ������Ҫ�豸��
��2������д����ҵ������������ʯ�ҷ�Ӧ��ȡƯ�۵Ļ�ѧ��Ӧ����ʽ�� ��
��3������д��θ��ƽ��������������������θ�ᣨ���ᣩ��������ӷ�Ӧ����ʽ�� ��
��4��ӡˢ��·�����ɸ߷��Ӳ��Ϻ�ͭ�����϶��ɣ�����ӡˢ��·��ʱ��Ҫ��FeCl3��Һ��Ϊ����ʴҺ����
��д��FeCl3��Һ����ʴ��ͭ�������ӷ���ʽ ��
��ij��ѧ��ȤС��Ϊ����ij���Ҹ�ʴ�Ժ����û����Һ��ɣ���̽�Ե���100 mL��ʴ��Ļ����Һ�м���2.80g���ۣ����ȫ���ܽ���δ������������������Һ�����Ϊ����д��ѧʽ����
��1��ʯ��ʯ��������¯ ��2��2Cl2+2Ca(OH)2= Ca(ClO)2 +CaCl2��2H 2O
��3��Al(OH)3+3H+= Al3+��3H 2 ��4����2Fe3++Cu=2Fe2++Cu2+ ��FeCl3��FeCl2��CuCl2
��3��Al(OH)3+3H+= Al3+��3H 2 ��4����2Fe3++Cu=2Fe2++Cu2+ ��FeCl3��FeCl2��CuCl2
�����������1��������������Ҫԭ���Ǵ��ʯ��ʯ��ʯӢ������ˮ�����Ҫԭ���������ʯ��ʯ����������Ҫԭ��������ʯ����̿��ʯ��ʯ�Ϳ��������Զ�Ҫ�õ���ԭ����ʯ��ʯ����ҵ��������Ҫ�豸�Ǹ�¯��
��2����������ʯ����Ư�۵ķ���ʽΪ2Cl2+2Ca(OH)2= Ca(ClO)2 +CaCl2��2H 2
��3��θ��ƽ����θ���������ӷ���ʽΪAl(OH)3+3H+= Al3+��3H 2
��4����FeCl3��Һ����ʴ��ͭ�������ӷ���ʽ2Fe3++Cu=2Fe2++Cu2+����ʴ�����Һ�ܼ���2.80g���ۣ�û�й���������˵��Cu2+û�в��뷴Ӧ��������Һ�д���Fe3+��������Һ�����ΪFeCl3��FeCl2��CuCl2��
����������dz���������Ҫ���鷽��ʽ����д��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ




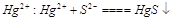
 ͨ��Ca(ClO)2��Һ�У�Ca2++2C1O
ͨ��Ca(ClO)2��Һ�У�Ca2++2C1O +SO2=====CaSO4��+2C1
+SO2=====CaSO4��+2C1