��Ŀ����
���ڴ�ŵ��������ƿ��ܻᱻ������ij��ѧ����С����ͨ��ʵ����ȷ��ij��ˮ�������Ʊ��ʣ����ⶨ�������ƵĴ��ȡ�
������λѧ����ȡ������Ʒ�������������ʵ�鷽����������Ϊ����۲쵽��������Լ���Ƶķ���һ�£�����ȷ֤��Һ����SO42�����ӡ�����Ϊ�����ķ����� ��
�����ף���Һ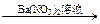 ��ɫ����
��ɫ����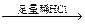 ��ɫ������
��ɫ������
�����ң���Һ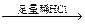 ����ɫ��������
����ɫ��������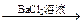 ��ɫ������
��ɫ������
����������Һ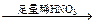 ��ɫ��Һ
��ɫ��Һ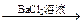 ��ɫ������
��ɫ������
��ͬѧ��ȡ35g����Ʒ������800mLijŨ�ȵ����ᣬ����ʹ���ɵ�����ȫ���ݳ������������ð�ˮ���գ���Һ����16g��Ȼ����ԭ��Һ����μ���0.5mol/L��Ba(OH)2��Һ500mL���г������ɣ���ʱ��Һ�����ԡ�
(1)�ð�ˮ�������巢����Ӧ�Ŀ��ܵ����ӷ���ʽ
��
(2)��Ʒ��Na2SO3�Ĵ����� ����������ʵ���Ũ��________________��
������λѧ����ȡ������Ʒ�������������ʵ�鷽����������Ϊ����۲쵽��������Լ���Ƶķ���һ�£�����ȷ֤��Һ����SO42�����ӡ�����Ϊ�����ķ����� ��
�����ף���Һ
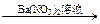 ��ɫ����
��ɫ����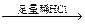 ��ɫ������
��ɫ�����������ң���Һ
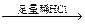 ����ɫ��������
����ɫ��������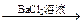 ��ɫ������
��ɫ����������������Һ
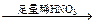 ��ɫ��Һ
��ɫ��Һ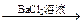 ��ɫ������
��ɫ��������ͬѧ��ȡ35g����Ʒ������800mLijŨ�ȵ����ᣬ����ʹ���ɵ�����ȫ���ݳ������������ð�ˮ���գ���Һ����16g��Ȼ����ԭ��Һ����μ���0.5mol/L��Ba(OH)2��Һ500mL���г������ɣ���ʱ��Һ�����ԡ�
(1)�ð�ˮ�������巢����Ӧ�Ŀ��ܵ����ӷ���ʽ
��
(2)��Ʒ��Na2SO3�Ĵ����� ����������ʵ���Ũ��________________��
(��8��) ����(2��)
��(1)2NH3��H2O+SO2(����)=2NH4++SO32��+H2O NH3��H2O+SO2(����)=NH4++HSO3��(��1��)
(2)90��(2��) (3)1.25mol/L(2��)
��(1)2NH3��H2O+SO2(����)=2NH4++SO32��+H2O NH3��H2O+SO2(����)=NH4++HSO3��(��1��)
(2)90��(2��) (3)1.25mol/L(2��)
��

��ϰ��ϵ�д�
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ