��Ŀ����
��18�֣��±�ΪԪ�����ڱ���һ���֣������г�11��Ԫ�������ڱ��е�λ�ã���Ҫ��ش����и��⡣

 ��1����11��Ԫ���У���ѧ��������õ�Ԫ���� ����Ԫ�ط��Ż�ѧʽ��ʾ,��ͬ�����õ���������ǿ��ԭ�Ӷ�Ӧ�ĵ����� ,ʧ����������ǿ��ԭ�Ӷ�Ӧ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��������ΪҺ̬�ķǽ��������� ��
��1����11��Ԫ���У���ѧ��������õ�Ԫ���� ����Ԫ�ط��Ż�ѧʽ��ʾ,��ͬ�����õ���������ǿ��ԭ�Ӷ�Ӧ�ĵ����� ,ʧ����������ǿ��ԭ�Ӷ�Ӧ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��������ΪҺ̬�ķǽ��������� ��
��2��Ԫ�آܵ����ӽṹʾ��ͼΪ ��
��3���ޡ��ߡ�����̬�⻯��Ļ�ѧʽ�ֱ�Ϊ �� �� �������� ���ȶ����ߡ�������Ԫ������������Ӧˮ����Ļ�ѧʽ�ֱ�Ϊ�� �� �������� ������ǿ��
��4���ۺ͢�����Ԫ���γɵĻ��������� (����ӻ�������ۻ����),���õ���ʽ��ʾ���γɹ��� ��
��5��д���۵ĵ����û����ĵ��ʵĻ�ѧ����ʽ ��

| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | | | �� | �� |
| 3 | �� | �� | �� | | �� | | �� | |
| 4 | �� | �� | | | | | 11 | |
 ��1����11��Ԫ���У���ѧ��������õ�Ԫ���� ����Ԫ�ط��Ż�ѧʽ��ʾ,��ͬ�����õ���������ǿ��ԭ�Ӷ�Ӧ�ĵ����� ,ʧ����������ǿ��ԭ�Ӷ�Ӧ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��������ΪҺ̬�ķǽ��������� ��
��1����11��Ԫ���У���ѧ��������õ�Ԫ���� ����Ԫ�ط��Ż�ѧʽ��ʾ,��ͬ�����õ���������ǿ��ԭ�Ӷ�Ӧ�ĵ����� ,ʧ����������ǿ��ԭ�Ӷ�Ӧ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��������ΪҺ̬�ķǽ��������� ����2��Ԫ�آܵ����ӽṹʾ��ͼΪ ��
��3���ޡ��ߡ�����̬�⻯��Ļ�ѧʽ�ֱ�Ϊ �� �� �������� ���ȶ����ߡ�������Ԫ������������Ӧˮ����Ļ�ѧʽ�ֱ�Ϊ�� �� �������� ������ǿ��
��4���ۺ͢�����Ԫ���γɵĻ��������� (����ӻ�������ۻ����),���õ���ʽ��ʾ���γɹ��� ��
��5��д���۵ĵ����û����ĵ��ʵĻ�ѧ����ʽ ��
��1��Ne F2 2K+2H2O=2KOH+H2�� Br2
��2��
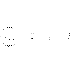
��3��CH4 PH3 HF HF H3PO4 HClO4 HClO4
��4�����ӻ�����
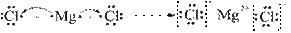
��5��2Mg+CO2
 2MgO+C
2MgO+C��

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
 2CA3(g)������Ӧ�ﵽƽ��ʱ���ϸı����������ı�A2��C2��CA3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������CA3�ĺ�����ߵ�һ��ʱ����____________���¶�ΪT ��ʱ����4a mol A2��2a mol C2�����ܱ������У���ַ�Ӧ����ƽ���
2CA3(g)������Ӧ�ﵽƽ��ʱ���ϸı����������ı�A2��C2��CA3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������CA3�ĺ�����ߵ�һ��ʱ����____________���¶�ΪT ��ʱ����4a mol A2��2a mol C2�����ܱ������У���ַ�Ӧ����ƽ��� ������CA3���������Ϊ50�������ʱC2��ת����Ϊ______________��
������CA3���������Ϊ50�������ʱC2��ת����Ϊ______________��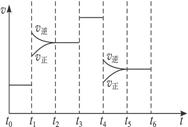
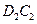 ����Y��Ӧ���ɵ���C���÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
����Y��Ӧ���ɵ���C���÷�Ӧ�Ļ�ѧ����ʽΪ____________________��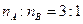 ����W�Ŀռ乹��Ϊ_______________����ҵ�Ϻϳ�W����ѡ���������________________��
����W�Ŀռ乹��Ϊ_______________����ҵ�Ϻϳ�W����ѡ���������________________��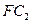 �����3mol C�����嵥�ʣ�һ�������·�Ӧ������
�����3mol C�����嵥�ʣ�һ�������·�Ӧ������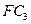 ���壬����Ӧ�ﵽƽ��ʱ������C��Ũ��Ϊ
���壬����Ӧ�ﵽƽ��ʱ������C��Ũ��Ϊ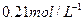 ����ƽ��ʱ
����ƽ��ʱ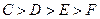
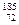 Hf������
Hf������
