��Ŀ����
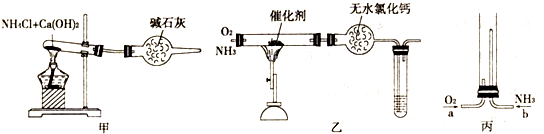
������ʾ�����������ڴ����а���ȼ�ա�����ijУ��ѧС��ѧ���������װ�ã�ͼ�����еȼг�װ������ȥ�����а����������ڲ�ͬ�����·�Ӧ��ʵ�顣
��1����װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ��__________________����ʯ�ҵ�������________________________________________________________________��
��2���������İ��������������ͨ��װ��B������Ϊ��ʯ�ޣ��У��þƾ���Ƽ��ȣ�
�ٰ��������Ļ�ѧ����ʽ��____________________________________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
��ֹͣ��Ӧ�������ر�B������������һ��ʱ����Թܽ����ˮ��������һ�ֶ�����NO2��Է��������Ļ�����Թ���������ɫ��dz�����ϻ�ѧ����ʽ˵��ԭ��__________
_____________________________________________________________________��
��3����������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У���b���϶˵�ȼ����δ������ɫ���壺
��������ͨ����Ⱥ�˳����_______________����������__________________________��
�ڰ���ȼ�յĻ�ѧ����ʽ��________________________________________________��
˼·��������1���ư�����̼���κ��������ǣ�NH4��2CO3��NH4HCO3����NH4��2CO3��NH4HCO3���ȷֽ�Ļ�ѧ����ʽΪ��![]() ��
��![]()
��Ȼ����ʯ�ҵ�����������CO2��H2O��
��2���ٰ��������ڲ�������������ȼ�գ�������NO��H2O�����ǰ�������������ĵ�һ��������������Ҳ˵������һ�㡣
![]()
�Թ��ڱ����ɫ���϶���NO�������ɵ�NO2��2NO+O2![]() 2NO2
2NO2
�ڿɴ��Ʋ⣬������NO2��Է��������Ļ�������N2O4������������ɫ�ģ����ҵ��������������ɡ��Թ�����ɫ��dz��˵��NO2������ȫת��ΪN2O4������NO2����N2O4�ķ�Ӧ����ʽӦ�ǣ�
2NO2![]() N2O4��
N2O4��
��3����Ӧ��ͨ����������ͨ�백�� �������Ե�ȼ�����Ұ�������Ⱦ����
�ڰ���û�д����������ȼ�գ���δ���к���ɫ�������ɣ��϶���������NO����Ӧ���ǵ�����4NH3+3O2![]() 2N2+6H2O
2N2+6H2O
�𰸣���1����NH4��2CO3��NH4HCO3 ![]() ��
��![]()
��2���� ![]()
2NO+O2![]() 2NO2
2NO2
�ڿɴ��Ʋ⣬������NO2��Է��������Ļ�������N2O4������������ɫ�ģ����ҵ��������������ɡ��Թ�����ɫ��dz��˵��NO2������ȫת��ΪN2O4������NO2����N2O4�ķ�Ӧ����ʽӦ�ǣ�
2NO2![]() N2O4��
N2O4��
��3����Ӧ��ͨ����������ͨ�백�� �������Ե�ȼ�����Ұ�������Ⱦ������
��4NH3+3O2![]() 2N2+6H2O
2N2+6H2O

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�