��Ŀ����
��ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ش���1����ϩ�ķ���ʽ�� ��
��2��������������������ϩ���Լ��� ��A��ˮ B�� ϡ���� C��������Ȼ�̼��Һ D�����Ը��������Һ
��3����һ�������£���ϩ����ˮ��Ӧ�����л���A�� A�Ľṹ��ʽ�� ��
��4���л���A�����ᶼ�������г������л��A�����ᷴӦ�Ļ�ѧ����ʽ�ǣ���Ӧ����Ϊ ��
��5��ijͬѧ��ѧϰA��֪ʶ�����������ʵ�飮��������Ϊ��
�����Թ������2mL A��
�ڰ�һ���������״��ͭ˿���ھƾ��������м��ȣ�
��������ͭ˿����ʢ��A���Թ�������������Σ�
�������������ζ���۲�ͭ˿����ı仯��
�Իش��������⣺
��1����ʵ���Ŀ���� ��
��2���ڢܲ������У�ͭ˿����ı仯�� ��
��3��д����Ӧ�Ļ�ѧ����ʽ ��
���𰸡���������1����ϩ�Ǻ���̼̼˫�������ϩ������д��ѧʽ��
��2����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ�����Ա�ǿ�����������������鲻�ܣ�
��3����ϩ����ˮ�����ӳɷ�Ӧ�õ��Ҵ���
��4���Ҵ��������ᷢ��������Ӧ�õ�����������ˮ��
��5���ٸ�ʵ��Ϊ�Ҵ��Ĵ�������Ӧ��
���Ҵ������ɫ������ͭ��Ӧ�õ���ȩ�ͺ�ɫ��ͭ�Լ�ˮ��
�۸����Ҵ���������ͭ��Ӧ�õ���ȩ��ͭ�Լ�ˮ��д��ѧ����ʽ��
����⣺��1����ϩ����һ��̼̼˫�������Է���ʽΪ��C2H4���ʴ�Ϊ��C2H4��
��2����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ��ʹ��ˮ��ɫ�����Ա�ǿ��������������������Ӷ�ʹ���������ɫ�������鲻�ܣ��ʴ�Ϊ��CD��
��3����ϩ���Ժ�ˮ�ӳ������Ҵ���H2C=CH2+H2O��CH3CH2OH���ʴ�Ϊ��C2H5OH��CH3CH2OH��
��4���Ҵ��������ᷢ��������Ӧ�õ�����������ˮ��CH3COOH+CH3CH2OH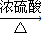 CH3COOCH2CH3+H2O������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
CH3COOCH2CH3+H2O������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��5���ٸ�ʵ��Ϊ�Ҵ��Ĵ�������Ӧ�����Ը�ʵ���Ŀ�����Ҵ��Ĵ�������Ӧ���ʴ�Ϊ���Ҵ��Ĵ�������Ӧ��
���Ҵ������ɫ������ͭ��Ӧ�õ���ȩ�ͺ�ɫ��ͭ�Լ�ˮ������ͭ˿�����ɺ�ɫ��ɺ�ɫ���ʴ�Ϊ��ͭ˿�����ɺ�ɫ��ɺ�ɫ��
���Ҵ���������ͭ��Ӧ�õ���ȩ��ͭ�Լ�ˮ��CH3CH2OH+CuO CH3CHO+H2O+Cu��
CH3CHO+H2O+Cu��
�ʴ�Ϊ��CH3CH2OH+CuO CH3CHO+H2O+Cu��
CH3CHO+H2O+Cu��
������������Ҫ����ѧ����ϩ���Ҵ��Ļ�ѧ���ʣ����Ը��ݽ̲�֪ʶ���ش��ѶȲ���
��2����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ�����Ա�ǿ�����������������鲻�ܣ�
��3����ϩ����ˮ�����ӳɷ�Ӧ�õ��Ҵ���
��4���Ҵ��������ᷢ��������Ӧ�õ�����������ˮ��
��5���ٸ�ʵ��Ϊ�Ҵ��Ĵ�������Ӧ��
���Ҵ������ɫ������ͭ��Ӧ�õ���ȩ�ͺ�ɫ��ͭ�Լ�ˮ��
�۸����Ҵ���������ͭ��Ӧ�õ���ȩ��ͭ�Լ�ˮ��д��ѧ����ʽ��
����⣺��1����ϩ����һ��̼̼˫�������Է���ʽΪ��C2H4���ʴ�Ϊ��C2H4��
��2����ϩ�к���̼̼˫�������Է����ӳɷ�Ӧ��ʹ��ˮ��ɫ�����Ա�ǿ��������������������Ӷ�ʹ���������ɫ�������鲻�ܣ��ʴ�Ϊ��CD��
��3����ϩ���Ժ�ˮ�ӳ������Ҵ���H2C=CH2+H2O��CH3CH2OH���ʴ�Ϊ��C2H5OH��CH3CH2OH��
��4���Ҵ��������ᷢ��������Ӧ�õ�����������ˮ��CH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
CH3COOCH2CH3+H2O������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ����5���ٸ�ʵ��Ϊ�Ҵ��Ĵ�������Ӧ�����Ը�ʵ���Ŀ�����Ҵ��Ĵ�������Ӧ���ʴ�Ϊ���Ҵ��Ĵ�������Ӧ��
���Ҵ������ɫ������ͭ��Ӧ�õ���ȩ�ͺ�ɫ��ͭ�Լ�ˮ������ͭ˿�����ɺ�ɫ��ɺ�ɫ���ʴ�Ϊ��ͭ˿�����ɺ�ɫ��ɺ�ɫ��
���Ҵ���������ͭ��Ӧ�õ���ȩ��ͭ�Լ�ˮ��CH3CH2OH+CuO
 CH3CHO+H2O+Cu��
CH3CHO+H2O+Cu���ʴ�Ϊ��CH3CH2OH+CuO
 CH3CHO+H2O+Cu��
CH3CHO+H2O+Cu��������������Ҫ����ѧ����ϩ���Ҵ��Ļ�ѧ���ʣ����Ը��ݽ̲�֪ʶ���ش��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ

 D�Ļ�ѧ��Ӧ����ʽ ��
D�Ļ�ѧ��Ӧ����ʽ ��
 B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
 B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��